November 18, 2013 — In an expert consensus document e-published in
Catheterization and Cardiovascular Interventions (
CCI), the Society for Cardiovascular Angiography and Interventions (SCAI) offers recommendations for optimal use of three technologies that interventional cardiologists often use to assess the severity of blockages in heart arteries. To ensure these technologies are applied in a manner most beneficial to patients, SCAI has issued guidance on
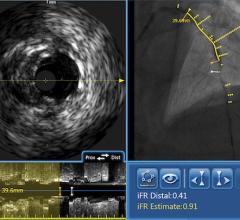
fractional flow reserve (FFR),
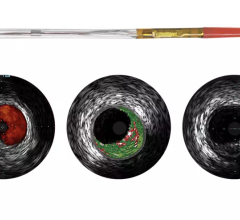
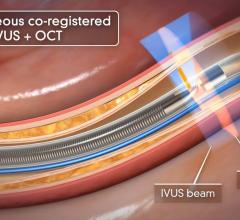
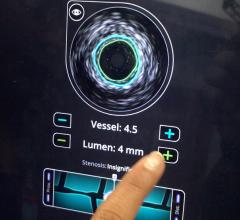
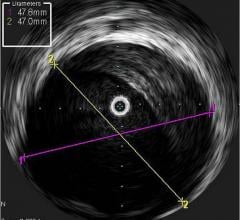
intravascular ultrasound (IVUS) and
optical coherence tomography (OCT), three tools for improved patient care, particularly for patients whose heart disease is considered complex or whose disease levels are unclear from angiography.
“Physicians will find these recommendations helpful as they apply these techniques to help patients with more complex cases or who have tests that contradict one another,” said Lloyd Klein, M.D., FSCAI, professor of medicine, Rush University Medical Center, Chicago and lead author. “Many patients will not need these techniques in their care, but for those who will benefit from them, this guidance offers physicians the parameters to ensure they are put to use best.”
General information
FFR is a procedure for measuring how much blood flow is restricted by a blockage in an artery using a pressure-sensing guide wire fed through a catheter to the site of the blockage in an artery. When placed across the blockage, the wire measures the flow and pressure of blood before and after the blockage. IVUS, a form of ultrasound, uses sound waves to create images of the inside of arteries to determine if a blockage is present, and if so, how severe the blockage is. OCT is an imaging method for taking high-resolution pictures of blood vessel walls. Approved in 2010 for cardiac use in the United States, OCT provides detailed images of build-up of cholesterol and other materials in blood vessel walls that can rupture, causing a blood clot to form and block off critical blood flow. All three techniques are typically applied during an angiogram to help interventional cardiologists assess the severity of the disease inside heart arteries.
The expert consensus document reviews each of the three technologies in terms of level of benefit to patients. This categorization offers physicians perspective on the best current uses of each test, where no proven benefit has been shown and where there may be potential for benefit once more information is gathered over time. Highlights include:
Benefits of FFR
- In patients with complex heart disease, angioplasty with FFR measurement improves outcomes and saves resources when compared to angioplasty guided by angiography alone.
- Knowing the FFR measurement may help guide decisions on whether to perform angioplasty or open-heart bypass surgery and whether a patient needs urgent in-hospital care to open blocked arteries versus medication alone.
Benefits of IVUS
- IVUS helps to accurately determine how well a stent is placed in an artery, ensuring a proper fit based on the size of the artery.
- IVUS can be used with certain types of blockages to help determine whether a patient will need revascularization to open a blocked artery. The authors note that IVUS should not be used to determine the severity of certain types of heart disease.
Benefits of OCT
- OCT is beneficial to determine how well a stent is placed, with improved imaging compared to IVUS, and can be useful in assessing changes in plaque that block the arteries.
The paper, titled “Expert Consensus Statement on the Use of Fractional Flow Reserve, Intravascular Ultrasound, and Optical Coherence Tomography” is available at on SCAI’s website.
For more information: www.scai.org



 September 18, 2025
September 18, 2025