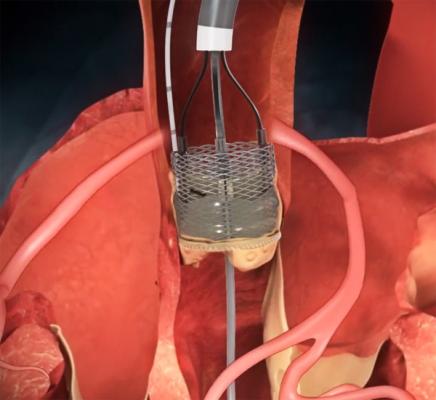
November 4, 2013 — In a
clinical trial of the Boston Scientific Lotus valve, a second-generation
transcatheter aortic valve, the device demonstrated low rates of complications that are sometimes seen in transcatheter aortic valve replacement (TAVR), including challenges with positioning, post-procedure paravalvular aortic regurgitation, vascular complications and stroke.
The findings were presented at the 25th annual Transcatheter Cardiovascular Therapeutics scientific symposium (TCT 2013).
The valve studied in REPRISE II is fully retrievable and repositionable with an adaptive seal intended to minimize paravalvular regurgitation, a complication that has been associated with higher mortality among patients undergoing TAVR. In this prospective, single-arm, multicenter study, symptomatic patients at high risk for surgery received the Lotus valve to treat calcific aortic stenosis.
The trial enrolled 120 patients; mean age was 84.4±5.3 years, 56.7 percent were female and 75.8 percent were considered New York Heart Association (NYHA) Class III or IV. The mean Society of Thoracic Surgeons score was 7.1±4.6 percent and all patients were confirmed by their site heart team to be at high risk for surgery due to frailty or associated comorbidities.
The valve was successfully implanted in all 120 patients with valve repositioning and retrieval performed as needed. There was no embolization, ectopic valve deployment or need for implantation of a second prosthetic valve.
The primary device performance endpoint was the mean aortic valve pressure gradient at 30 days compared to a performance goal of 18 mmHg; the primary safety endpoint was 30-day mortality. The primary device performance endpoint was met with a 30 day mean aortic valve pressure gradient of 11.5±5.2 mmHg; mean effective orifice area was 1.7±0.4 cm2.
All cause mortality and disabling stroke were low at 30 days (4.2 percent and 1.7 percent, respectively). Additional clinical event rates were consistent with those reported for other valves. Aortic regurgitation at 30 days was negligible in 99 percent of patients (78.3 percent none, 5.2 percent trace and 15.5 percent mild). The total stroke rate, disabling and non-disabling, was 5.9 percent, which is the same as the rate as the Edward's Sapien valve's performance in the PARTNER trial.
“These findings suggest this valve, which is a differentiated, second generation TAVR device, will be a valuable addition for the treatment of severe aortic stenosis,” said Ian Meredith, MBBS, Ph.D., director, Monash HEART, executive director, Monash Cardiovascular Research Centre, professor of medicine, Monash University in Melbourne, Australia, and lead investigator of the study.
For more information: www.crf.org, www.bostonscientific.com