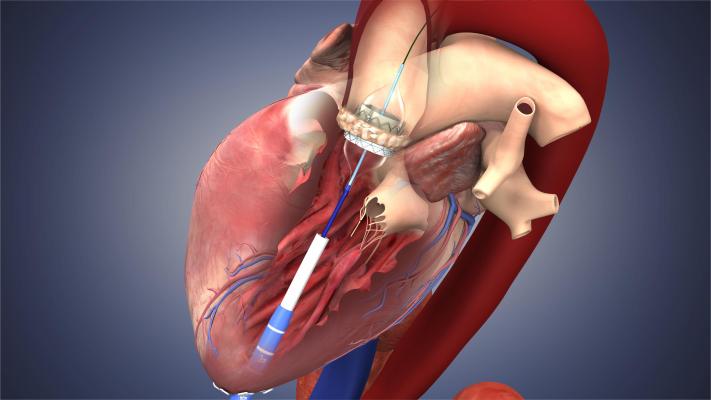
November 21, 2013 — Data from a national registry developed to track patient safety and real-world outcomes finds that the initial use of transcatheter aortic valve replacement (TAVR) for treatment of aortic stenosis in high surgical risk and inoperable patients in the United States is safe and effective. The data, published as a study in the Nov. 20 issue of the Journal of the American Medical Association (JAMA), confirms results of premarket clinical trials and represents the first public report from the STS/ACC (Society of Thoracic Surgeons/American College of Cardiology) Transcatheter Valve Therapy (TVT) registry.
“The large volume of data in the registry provided an excellent source to promptly examine patient characteristics and outcomes,” said Michael Mack, M.D., Heart Hospital Baylor Plano, Baylor Healthcare System, Plano, Texas, and chair of the STS/ACC TVT Registry Steering Committee and lead author.
The STS/ACC TVT registry was launched December 2011, shortly after U.S. Food and Drug Administration (FDA) approval of the Edwards Lifesciences Sapien transcatheter heart valve. In May 2012, the Centers for Medicare & Medicaid Services (CMS) required all hospitals performing TAVR to capture clinical information in the STS/ACC TVT Registry as a requirement for Medicare coverage. CMS uses hospital data to facilitate outcomes assessment, as well as comparison with other trials and international registries. Building the STS/ACC TVT registry was a joint effort of STS, ACC, FDA, CMS, the National Institutes of Health and Duke Clinical Research Institute. It represents the first time that such a broad collaborative group has supported the safe introduction and rational dispersion of new medical devices in the United States.
“The TVT registry is the result of two transformational events: The first is based on the approval and now widespread use of TAVR technology in the United States and around the world, and the second is the coming together of professional societies and U.S. regulatory agencies to optimize outcomes of patients with severe valvular heart disease,” said David Holmes, M.D., former president of the ACC. “These events promise to be increasingly important vehicles to help ensure that U.S. patients have efficient access to safe and effective new strategies of care. This first manuscript is the initial delivery of what these transformational events can accomplish.”
The analysis included 7,710 patients who underwent TAVR at one of 224 of the sites participating in the TVT registry. Among patients included in the analysis, device implantation was successfully achieved in 92 percent of operations. The overall in-hospital mortality rate was 5.5 percent and the stroke rate was 2 percent.
“Given the low in-hospital mortality and stroke rates, our real-world results from this newly introduced technology are similar to the success rates and complication patterns documented in carefully performed randomized trials that resulted in FDA approval,” said Mack. “The results are also similar to the global experience of TAVR, which is now based on second- and third-generation devices. We think that the American public can feel comfortable that this new registry is an effective means of monitoring the performance of new medical devices after they have been approved. ”
Mack cautioned, however, that longer term follow-up is essential to assess continued safety, efficacy and patient health status.
Future of the TVT Registry
The TVT registry currently has more than 10,000 patient records and enrollment is on track to double that number in the next year. The large number of patient records allows for the development of reliable benchmarks for centers to compare their local results against risk-adjusted national standards. Ultimately this will translate into improved patient selection and optimal patient care.
“We envision the TVT registry as an evolving portfolio of transcatheter heart valve device modules as part of the FDA’s new strategic plan for post-market device surveillance,” said Fred Edwards, M.D. director, STS Research Center and study author. “As new medical devices are approved in the United States, we intend to incorporate experience with these devices into the registry. This should provide a streamlined approach to national device surveillance and open up new avenues into research focused on the risks and benefits of medical device products.”
For more information: www.tvtregistry, jama.jamanetwork.com, www.sts.org, cardiosource.org


 January 05, 2026
January 05, 2026 









