
Figure 1: Positive MTWA test result
During a sudden cardiac arrest (SCA), heart function ceases – abruptly and without warning – most often due to the onset of a ventricular tachyarrhythmia [1]. Because out-of-hospital survival is less than 8 percent, prediction and prevention are critically important [2]. Microvolt T-wave alternans (MTWA), a subtle alternating pattern in T-wave morphology on the surface electrocardiogram is a marker of arrhythmic vulnerability and SCA risk [3, 4], demonstrating prognostic value in various patient populations including those with ischemic and non-ischemic cardiomyopathy [5-7]. We report a case of a patient who experienced SCA one year after a positive MTWA test.
Case History
An asymptomatic 64-year-old male had a history of coronary artery disease and coronary bypass surgery in 1992. A computed tomography (CT) angiogram performed in September 2006 revealed subtotal occlusion of the right coronary artery; however, angioplasty could not be completed due to anatomical complexity. An echocardiogram showed a left ventricular ejection fraction (LVEF) of 45-50 percent and significant left ventricular hypertrophy secondary to moderate aortic stenosis. Given his clinical profile, an MTWA test was ordered to further clarify his risk of ventricular arrhythmias and SCA.
MTWA Study and Follow-up
In October 2009, MTWA testing was performed with treadmill exercise using the Analytic Spectral Method to measure alternans (HearTwave II, Cambridge Heart, Tewksbury, Mass.). At the time of the test, the patient was in normal sinus rhythm with intermittent ventricular ectopy. The MTWA result was positive with sustained alternans present in several leads with an onset heart rate of 106 bpm (Fig. 1). The positive MTWA study along with the patient’s other risk factors prompted an evaluation by an electrophysiologist.
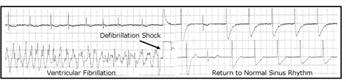
An electrophysiology study (EPS) was performed resulting in inducible ventricular tachycardia with two extrastimuli, and a dual-chamber implantable cardioverter defibrillator (ICD) (Secura DR, Medtronic, Minneapolis, Minn.) was implanted in November 2009. One year later while resting at home, the patient reported feeling a “flutter” in his heart followed by a shock from the device. Review of the ICD electrogram (Fig. 2) confirmed that the patient experienced an episode of ventricular fibrillation which was successfully terminated by a 23.7 J defibrillation shock on Dec. 2, 2010.
Discussion
Reduced left ventricular ejection fraction is an established risk factor for cardiac arrest and is currently the primary determinant of ICD eligibility; however, EF lacks sensitivity [8] due to the fact that the majority of SCA deaths occur in patients with an EF more than 30-35 percent [9, 10]. This patient did not have significant LV dysfunction, but had several other clinical factors associated with increased risk of SCA including ischemic cardiomyopathy [11], left ventricular hypertrophy [12] and aortic stenosis [13].
MTWA has been cited as a useful adjunct tool in such patients with moderate to preserved LVEF for whom SCA is a “real but difficult-to-ascertain risk” [14]. MTWA can help guide SCA risk management strategies which may include treatment of underlying ischemia, medical optimization, tighter glucose control, lifestyle changes – or, when appropriate, EPS referral and possible EPS or ICD implantation.
“Per current primary prevention guidelines, this patient would not have received life-saving ICD therapy if he had not undergone an MTWA study,” noted Keith Boman, M.D., the patient’s cardiologist. “MTWA is a tool to help clarify the overall arrhythmic risk, particularly for patients who have known risk factors but who fall into this ‘gray area’ in terms of ICD eligibility.”
References
1. Bayes de Luna A, Coumel P, and Leclercq JF. "Ambulatory sudden cardiac death: mechanisms of production of fatal arrhythmia on the basis of data from 157 cases." Am Heart J, 1989; 117(1): 151-9.
2. Lloyd-Jones D, Adams RJ, Brown TM, et al. "Heart disease and stroke statistics--2010 update: a report from the American Heart Association." Circulation; 121(7): e46-e215.
3. Pastore JM, Girouard SD, Laurita KR, Akar FG, and Rosenbaum DS. "Mechanism linking T-wave alternans to the genesis of cardiac fibrillation." Circulation, 1999; 99(10): 1385-94.
4. Rosenbaum DS, Jackson LE, Smith JM, Garan H, Ruskin JN, and Cohen RJ. "Electrical Alternans and Vulnerability to Ventricular Arrhythmias." N Engl J Med, 1994; 330(4): 235-241.
5. Chow T, Kereiakes DJ, Bartone C, et al. "Prognostic utility of microvolt T-wave alternans in risk stratification of patients with ischemic cardiomyopathy." J Am Coll Cardiol, 2006; 47(9): 1820-7.
6. Salerno-Uriarte JA, De Ferrari GM, Klersy C, et al. Prognostic Value of T-Wave Alternans in Patients With Heart Failure Due to Nonischemic Cardiomyopathy: Results of the ALPHA Study. J Am Coll Cardiol 2007; 50(19): 1896-1904.
7. Bloomfield DM, Bigger JT, Steinman RC, et al. "Microvolt T-wave alternans and the risk of death or sustained ventricular arrhythmias in patients with left ventricular dysfunction." J Am Coll Cardiol, 2006; 47(2): 456-63.
8. Buxton AE, Lee KL, Hafley GE, et al. "Limitations of ejection fraction for prediction of sudden death risk in patients with coronary artery disease: lessons from the MUSTT study." J Am Coll Cardiol, 2007; 50(12): 1150-7.
9. de Vreede-Swagemakers JJ, Gorgels AP, Dubois-Arbouw WI, et al. "Out-of-hospital cardiac arrest in the 1990's: a population-based study in the Maastricht area on incidence, characteristics and survival." J Am Coll Cardiol, 1997; 30(6): 1500-5.
10. Stecker EC, Vickers C, Waltz J, et al. "Population-based analysis of sudden cardiac death with and without left ventricular systolic dysfunction: two-year findings from the Oregon Sudden Unexpected Death Study." J Am Coll Cardiol, 2006; 47(6): 1161-6.
11. Huikuri HV, Castellanos A, and Myerburg RJ. "Sudden death due to cardiac arrhythmias." N Engl J Med, 2001; 345(20): 1473-82.
12. Haider AW, Larson MG, Benjamin EJ, and Levy D. "Increased left ventricular mass and hypertrophy are associated with increased risk for sudden death." J Am Coll Cardiol, 1998; 32(5): 1454-9.
13. Kulbertus HE. "Ventricular arrhythmias, syncope and sudden death in aortic stenosis." European Heart Journal 1988; 9(suppl E): 51-52.
14. Mirro MJ. "Strategies for reducing sudden cardiac death: Application of microvolt T-wave alternans testing in clinical practice." Heart Rhythm, 2009; 6(3): S45-S48.
This case study was supplied by Cambridge Heart
For more information: www.cambridgeheart.com


 January 15, 2026
January 15, 2026 









