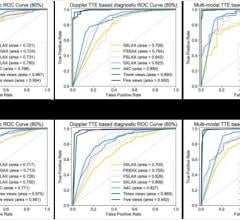
April 8, 2013 — The American Society of Echocardiography (ASE) has released a list of five interventions whose appropriateness physicians and patients should discuss as part of Choosing Wisely, an initiative of the ABIM Foundation, along with Consumer Reports. Fifth on the list, they ask that patients and their doctors talk about the real need for a transesophageal echocardiography (TEE) if the patient’s clot treatment is not likely to be modified.
Avoid TEE to detect cardiac sources of embolization, if a source has been identified and patient management will not change.
TEE involves taking echo pictures from within the body to allow the imaging device to be placed directly next to the heart, so the quality of the images is enhanced and allows for identification of possible sources of a stroke including blood clots within the heart. Placing the imaging probe into the mouth and then advancing it into the esophagus of the patient on whom it is performed, is not without risk. In order to do this safely and comfortably, sedatives are administered and a local anesthetic is gargled in the mouth. Both the administration of these medications and placing of the probe in the esophagus are not entirely benign procedures, and, while complications rarely occur, there is a possibility of damage to the esophagus and choking or aspiration. Because of these risk factors, the procedure should only be done when the care of the patient will possibly change based upon the result.
When clots are thought to be the cause of a stroke and their source has been identified, blood thinners are usually started to prevent a second stroke. In some cases, doctors make the decision to use blood thinners such as warfarin or Coumadin even when there is no definite clot found. In other cases, these blood thinners are contraindicated due to a problem with bleeding. In both of these scenarios, further information about the cardiac source would not change the treatment thus even the small risk of the TEE is not justified.
For more information: www.choosingwisely.org, www.asecho.org


 June 13, 2024
June 13, 2024