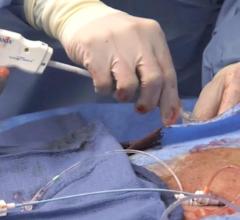
There is no shortage of debate among interventional cardiologists over whether vascular closure devices should be used for standard cath lab procedures. However, some say these devices have a definite niche in areas where they were not originally intended but have found frequent off-label use because of their utility.
The larger sizes of devices used for transcatheter endovascular aortic repair (TEVAR) and transcatheter aortic valve replacement (TAVR) requires a much larger arteriotomy site than standard percutaneous coronary interventions (PCI). However, these larger holes also make it more difficult to achieve hemostasis using manual compression alone.
Some of these devices are as large as 24 French, and anything larger requires a surgical cut-down, said Chris Malaisrie, M.D., assistant professor of surgery, division of cardiac surgery, Northwestern University Memorial Hospital, Chicago. “You can’t just hold pressure on a hole that size,” Malaisrie explained. “I use the preclose technique for large bore access for TEVAR and TAVI. I currently use Abbott Prostar or Abbott Perclose Proglide off-label. I prefer the ProGlide, even though you need two of them to close.”
The Abbott Perclose ProGlide suture-mediated closure system provides a suture for vascular closure of 5 – 21 French femoral artery access sites. The vendor says for sheath sizes greater than 8 French, at least two devices and the pre-close technique are required. The system helps reduce time to hemostasis, ambulation and discharge.
Another off-label application of these devices has been found when using percutaneous ventricular assist devices (pVADs), such as the Abiomed Impella or the CardiacAssist TandemHeart systems. The TandemHeart system uses cannula sizes up to 17 French.
“We primarily use two ProGlides for Impella 13 French closure using an off-label pre close technique,” said Mladen Vidovich, M.D., FACC, FSCAI, chief, section of cardiology, Jesse Brown VA Medical Center, and associate professor of medicine, University of Illinois, Chicago.
He said cath labs at both Jesse Brown VA and UIC used closure devices on a regular basis for femoral access PCI in the past to speed patient ambulation and cut nursing time. However, he said both facilities have drastically reduced the need for these devices.
“Due to our high radial access volume, our closure device use has dwindled, but for the rare groin I’ll use Proglide,” Vidovich said.


 October 27, 2021
October 27, 2021