March 24, 2014 — Sorin Group has purchased Oscor Inc. lead business, including a lead manufacturing facility in the ...
Leads Implantable Devices
News and new technology innovations for leads implantable devices can be found on this channel.
March 21, 2014 — Boston Scientific Corp. received CE marking and launched in Europe the Ingevity family of magnetic ...
March 6, 2014 — Biotronik announced the first implantations of the Sentus ProMRI lead. Biotronik’s bipolar cardiac ...


June 6, 2013 — Data presented at Heart Rhythm 2013 shows that Medtronic Inc. Lead Integrity Alert (LIA) software ...
May 13, 2013 — The Population Health Research Institute (PHRI) has conducted a further independent analysis of data ...
May 6, 2013 — St. Jude Medical announced its first enrollment in its MultiPoint Pacing clinical study to build upon its ...
April 30, 2013 — St. Jude Medical Inc. announced CE Mark approval and European launch of its Allure Quadra Cardiac ...
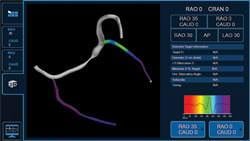
April 10, 2013 — Medtronic Inc. announced market release of the CardioGuide Implant System, a novel real-time navigation ...
March 18, 2013 — Medtronic Inc. announced it has received CE mark and will begin the European launch of the Attain ...
February 25, 2013 — The U.S. Food and Drug Administration (FDA) granted final approval for the Biotronik Lumax 740 DX ...
December 28, 2012 — The first patient has been implanted with the Boston Scientific Corporation next generation Ingevity ...
December 21, 2012 — Nanostim Inc. announced the first successful implants of a leadless pacemaker in a series of 11 ...

 March 24, 2014
March 24, 2014