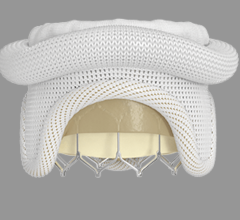
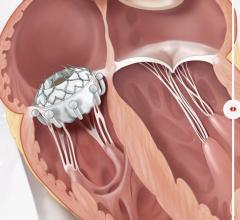
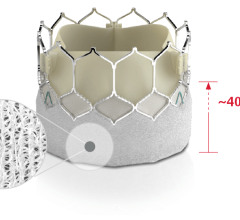
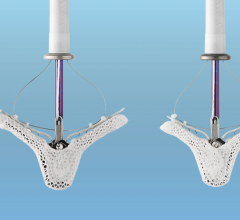
January 6, 2023 — Patients with congenital heart diseases often suffer from obstructions in the right ventricular ...
Heart Valve Technology
This channel includes news and new device innovations about heart valve technologies, including the aortic valve, mitral valve, pulmonic valve, and tricuspid valve. This includes information on transcatheter valve technologies like transcatheter aortic valve replacement (TAVR, or implantation TAVI), transcatheter mitral valve repair or replacement (TMVR), transcatheter and surgical valve repairs, and surgical replacement valves. Newer devices are now being used for transcatheter tricuspid valve repair replacement (TTVR).
January 2, 2023 — The U.S. Food and Drug Administration (FDA) has given the approval to market to The Edwards ...
December 29, 2022 — The Smidt Heart Institute at Cedars-Sinai was named a Mitral Valve Repair Reference Center, a ...
December 14, 2022 — Edwards Lifesciences identified the top data releases from 2022 that contributed most to shaping ...
December 13, 2022 — Edwards Lifesciences Corporation announced that one-year results on patients treated in the single ...
December 2, 2022 — Boston Scientific Corporation has announced the first results from the ACURATE neo2 Post Market ...
November 30, 2022 — Boston Scientific Corporation has announced the first results from the ACURATE neo2 Post Market ...
November 22, 2022 — CroíValve has announced the successful First in Human implants of its DUO Tricuspid Coaptation Valve ...
October 18, 2022 — Foldax, Inc. announced that medtech veteran Gregory D. Casciaro has been named Chief Executive ...
September 26, 2022 — Edwards Lifesciences announced the launch of the SAPIEN 3 Ultra RESILIA valve, which incorporates ...
September 15, 2022 — Cardiac Implants LLC has announced the successful initial deployment of its Annuloplasty ring with ...
September 9, 2022 — The U.S. Food and Drug Administration (FDA) is alerting healthcare providers about potential clip ...
August 4, 2022 — The Cardiovascular Research Foundation (CRF) has announced the late-breaking clinical science that will ...
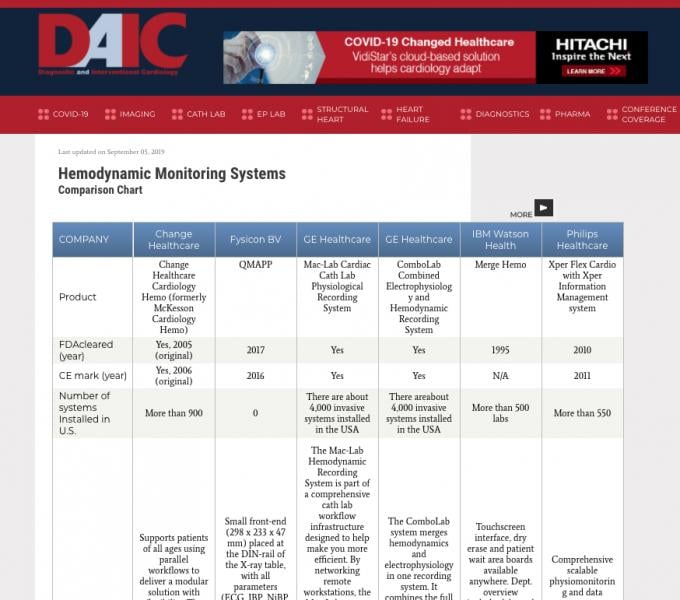
Diagnostic and Interventional Cardiology (DAIC) maintains more than 50 comparison charts of product specifications from ...

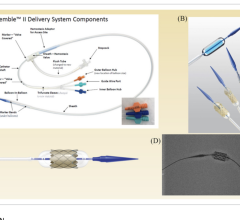
 January 06, 2023
January 06, 2023