December 17, 2014 – Medtronic Inc. announced the U.S. Food and Drug Administration (FDA) approval and commercial launch ...
Implantable Cardioverter Defibrillator (ICD)
This channel includes news and new technology innovations for implantable cardioverter defibrillators (ICD) used to treat tachycardia arrhythmias and heart failure. This includes cardiac resynchronization therapy defibrillators (CRT-D).
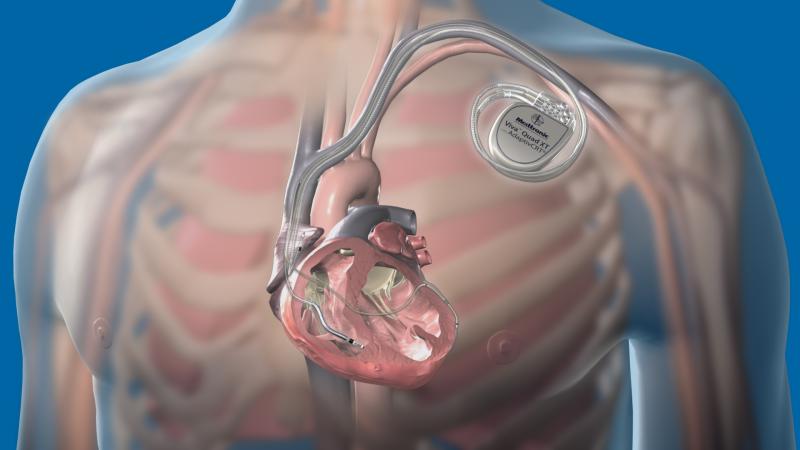
December 11, 2014 — A new study evaluating Optim-insulated implantable cardioverter defibrillator (ICD) leads found low ...
December 5, 2014 — Most patients with implantable cardioverter defibrillators (ICDs) haven’t thought about device ...
November 14, 2014 — Boston Scientific Corp. announced that its subcutaneous implantable defibrillator (S-ICD System) ...
September 12, 2014 — Ecolab Healthcare announced the availability of the PD220 patient drape, the first to market ...
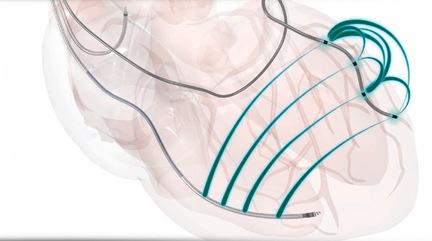
September 9, 2014 — Use of a quadripolar left ventricular (LV) lead instead of a bipolar option during cardiac ...
August 27, 2014 — Fysicon received approval from the U.S. Food and Drug Administration (FDA) to market Fysicon DataLinQ ...
August 11, 2014 — Medtronic announced it has received U.S. Food and Drug Administration (FDA) approval for the Attain ...
July 10, 2014 — Biotronik announced that the first device patients to undergo full-body magnetic resonance imaging (MRI) ...
May 28, 2014 — Biotronik announced that the U.S. Food and Drug Administration (FDA) has approved a significant expansion ...

May 23, 2014 — Medtronic announced the results from the first prospective randomized clinical trial to show that ...

May 22, 2014 — A study presented at Heart Rhythm 2014, the Heart Rhythm Society’s 35th Annual Scientific Sessions ...

May 19, 2014 — St. Jude Medical announced that data presented during a late-breaking clinical trial session at the 2014 ...

May 13, 2014 — The Heart Rhythm Society (HRS), American College of Cardiology (ACC) and American Heart Association (AHA) ...

 December 17, 2014
December 17, 2014