February 27, 2008 - Boston Scientific Corp. said the FDA approved three products in its Cardiac Rhythm Management ...
Implantable Cardioverter Defibrillator (ICD)
This channel includes news and new technology innovations for implantable cardioverter defibrillators (ICD) used to treat tachycardia arrhythmias and heart failure. This includes cardiac resynchronization therapy defibrillators (CRT-D).

When cardiologists send their high-risk patients home, they may be protected with a host of tools, including ...
February 4, 2008 – Fluke Biomedical unveiled today new defibrillator and pacemaker analyzer technologies, the ...
January 22, 2008 - Boston Scientific Corp. today announced CE Mark approval for its COGNIS cardiac ...
January 22, 2008 - The International Board of Heart Rhythm Examiners (IBHRE) issued the first standardized cardiac ...
January 8, 2007 - Cardiac Science Corp. expects 2007 fourth quarter revenue to total approximately $50 million ...
December 29, 2007 - Boston Scientific Corp. received CE Mark approval for its LIVIAN cardiac resynchronization ...
December 26, 2007 - Medtronic Inc. announced that it has entered into an agreement to settle lawsuits relating to ...
December 17, 2007 – Medtronic today announced it received Japanese regulatory approval on December 10, 2007 for the ...
December 4, 2007 - ELA Medical Inc. intiated U.S. enrollment in the OPTION study (Optimal Anti-tachycardia Therapy ...
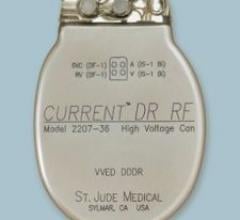
St. Jude Medical Inc. announced FDA approval of its first radiofrequency (RF) wireless devices to treat patients ...
November 13, 2007- There is significant underutilization of many guideline-indicated life-saving medical and ...
St. Jude Medical Inc. announced FDA approval of its first radiofrequency (RF) wireless devices to treat patients ...
November 7, 2007 - The application of clinical practice modifications combined with advanced electronic ...
October 16, 2007 – Medtronic Inc. said in a statement that it has voluntarily suspended worldwide distribution of ...

 February 26, 2008
February 26, 2008