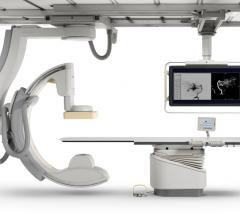
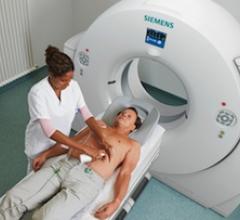
Cath lab volumes have gone down in recent years for several reasons, but one of the biggest reasons might be market ...
Interventional Radiology
Interventional radiology uses tools like embolization devices to provide minimally invasive medical diagnosis and treatment using images.
June 21, 2012 — Surefire Medical Inc. announced that it has received 510(k) U.S. Food and Drug Administration (FDA) ...
June 18, 2012 — St. Jude Medical Inc. announced it has received U.S. Food and Drug Administration (FDA) clearance and ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
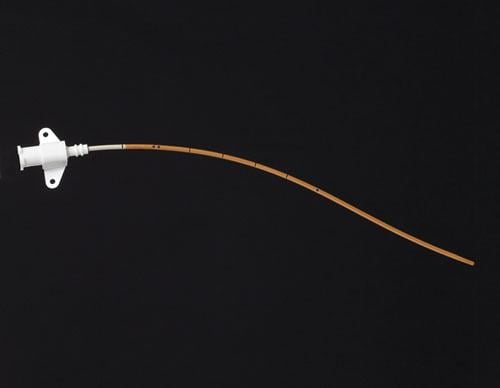
Peripherally inserted central catheters (PICCs) are devices used for intravenous access to facilitate the delivery of ...
May 30, 2012 — Boston Scientific Corp. announces the U.S. Food and Drug Administration has cleared an expanded use ...
May 1, 2012 — Theragenics Corp. announced that Galt Medical, a wholly-owned subsidiary of Theragenics, has received U.S ...
April 17, 2012 - Approximately 6 million Americans have brain aneurysms, a condition that occurs when a weak or thin ...
April 12, 2012 – Terumo Interventional Systems recently announced the nationwide availability of the Azur D35 Framing ...
April 12, 2012 — Philips Healthcare recently installed an Allura Xper FD 20/20 biplane angiography X-ray system at ...
April 6, 2012 — Crux Biomedical announced that a recently completed pivotal trial of its Crux Vena Cava Filter (VCF) ...
April 3, 2012 — New guidelines for CT-guided biopsies of lung nodules significantly reduce radiation exposure, allowing ...
April 2, 2012 — Interventional radiology treatments re-establish blood flow in people with chronic deep vein thrombosis ...
As catheter-based, minimally invasive procedures expand rapidly beyond treatment of the coronary arteries into all areas ...
February 16, 2012 — U.S. Food and Drug Administration (FDA) granted 510(k) clearance for AngioDynamics NeverTouch Direct ...
February 6, 2012 — Vascular Solutions Inc. announced it is marketing a reprocessing service for the ClosureFAST ...


 July 17, 2012
July 17, 2012