Sept. 16, 2025 — Elutia Inc., a pioneer in drug-eluting biomatrix technologies, has published clinical and preclinical data supporting the clinical utility of a biologic envelope that secures cardiac ...
Pacemakers
This channel includes news and new technology innovations for pacemakers used to treat bradycardia.
Sept. 16, 2025 — Elutia Inc., a pioneer in drug-eluting biomatrix technologies, has published clinical and preclinical ...
May 1, 2025 — Camgenium, a leading medical software engineering company, has announced further details of its ...
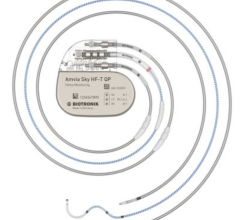
July 11, 2024 — Dr. Fadi Mansour performed the first Canadian implant of BIOTRONIK’s newest pacemaker and CRT-P ...
June 20, 2024 — A programming algorithm, being tested by HonorHealth Research Institute for those patients with new or ...
June 17, 2024 — Elutia Inc., a pioneer in drug-eluting biomatrix products, today announced that its Antibiotic-Eluting ...
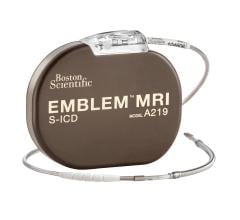
May 18, 2024 — Boston Scientific Corporation today announced positive six-month results from the ongoing pivotal MODULAR ...
May 15, 2024 — A new study demonstrated parity between a minimally invasive procedure to replace the aortic valve in the ...
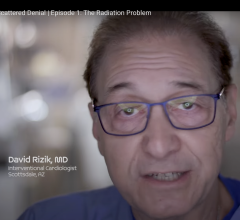
May 7, 2024 — HonorHealth Research Institute’s David G. Rizik, M.D., narrates and is a co-producer of a documentary ...
March 26, 2024 — UC San Diego Health is the first health system in San Diego to successfully implant the world’s first ...
March 15, 2024 — Following the recent CE approval of the Solia S lead1 for LBBAP, BIOTRONIK proudly announces the world ...
March 15, 2024 — Following the recent CE approval of the Solia S lead1 for LBBAP, Biotronik announces the world’s first ...
February 14, 2024 — The American College of Cardiology’s newest registry offers data-driven insights on cardiac ...
January 19, 2024 — Orchestra BioMed, a biomedical company accelerating high-impact technologies to patients through risk ...
January 11, 2024 — It is with great sadness that the Diagnostic and Interventional Cardiology (DAIC) team has learned of ...
January 5, 2024 — Medtronic, a global leader in healthcare technology, today announced it has received CE (Conformité ...





 September 19, 2025
September 19, 2025