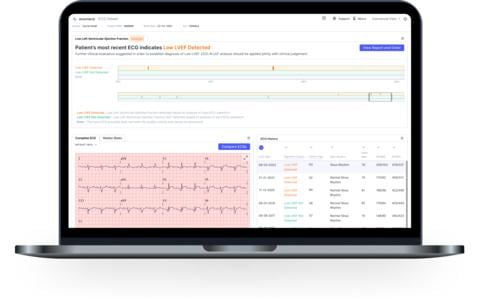
Screen shot of sample data from Anumana's ECG-AI LEF, a breakthrough artificial intelligence (AI)-powered medical device to detect low ejection fraction (LEF) in patients at risk of heart failure. Source: Anumana
October 4, 2023 — Anumana, Inc., a leading AI-driven health technology and nference portfolio company working in collaboration with Mayo Clinic, today announced U.S. Food and Drug Administration (FDA) 510(k) clearance for ECG-AI LEF, a breakthrough artificial intelligence (AI)-powered medical device to detect low ejection fraction (LEF) in patients at risk of heart failure.
LEF, or a weak heart pump, is a significant, at times asymptomatic, and commonly undiagnosed indicator of heart failure.1 The increasing prevalence of heart failure and its associated morbidity, mortality, rehospitalizations, and societal costs2,3 underscores the need to identify and manage patients with LEF.
“Anumana’s ECG-AI LEF fills an important unmet need – the lack of an easily accessible point-of-care, noninvasive, and inexpensive tool to screen for a weak heart pump,” said Paul Friedman, M.D., chair of the Department of Cardiovascular Medicine at Mayo Clinic in Rochester, Minn. and chair of Anumana’s Board of Advisors. “It allows identification of otherwise hidden disease, for which many effective, lifesaving treatments are available – once the presence of the disease is known.”
Developed in partnership with Mayo Clinic, Anumana’s ECG-AI LEF is an innovative software-as-a-medical device (SaMD) designed to screen for LEF in adults at risk for heart failure using data from a routine 12-lead electrocardiogram (ECG), a rapid and common test used in both primary and specialty care. Based on pioneering research from Mayo Clinic,4 the algorithm has been developed utilizing over 100,000 ECG and echocardiogram data pairs from unique patients and has been clinically tested in more than 25 studies involving more than 40,000 patients in the U.S. and internationally.5
Anumana’s ECG-AI LEF was clinically validated in a multi-site, retrospective clinical study of 16,000 racially diverse patients, achieving its primary endpoint with an 84.5% sensitivity and 83.6% specificity.6 ECG-AI LEF achieved an AUROC7 of 0.932, demonstrating an ability to differentiate between LEF and ejection fraction (EF) >40% extremely well (a score ≥0.90 is considered excellent8) and better than most tests currently used in heart failure standard of care.9
Additionally, the EAGLE study, a groundbreaking prospective, randomized, controlled clinical trial by Mayo Clinic evaluated the use of an investigational version of the algorithm in routine clinical care of 22,641 adults by 120 primary care teams from 45 clinics or hospitals, demonstrating that ECG-AI LEF implementation improved clinician’s ability to diagnosis of LEF by 31% versus standard of care without increasing the overall rate of echocardiogram usage.10
ECG-AI LEF is one of Anumana’s broad pipeline of algorithms, including three additional FDA breakthrough device designation algorithms (pulmonary hypertension, cardiac amyloidosis, and hyperkalemia), and is founded on more than six years of pioneering ECG-AI research and development at Mayo Clinic, including nearly 100 peer-reviewed publications to date.
“Anumana was established in 2021 by nference in partnership with Mayo Clinic to unlock the electrical language of the heart through deep learning and improve disease diagnosis and patient care,” said Murali Aravamudan, co-founder and CEO of Anumana and nference. “In the short time of two years we have secured multiple FDA breakthrough device designations, entered multi-year agreements with three pharma partners, successfully established two new medical procedure codes for ECG AI technology, and now achieved our first FDA breakthrough medical device clearance. This is a significant milestone, and we are excited about the next phase of the journey, deploying our technology in the U.S. and globally to empower clinicians and enhance real-world clinical care.”
Anumana is focused on driving fast-paced adoption of the new ECG-AI category, clinically developing and commercializing its novel technologies in healthcare. The newly FDA-cleared ECG-AI LEF can be easily integrated with various ECG information management systems or directly with a patient’s electronic health record via Anumana’s web-based ECG Viewer to support clinical decision-making.
Anumana spearheaded the effort to bring reimbursement to ECG-AI, receiving approval for two Category III CPT codes from the American Medical Association in 2022. These codes are now available and designed to facilitate the use, adoption, and potential reimbursement of emerging technologies in clinical workflows.
For more information: anumana.ai


 February 03, 2026
February 03, 2026 









