November 23, 2010 – A device for peripheral transluminal angioplasty (PTA) and for capturing and containing embolic material during angioplasty in the lower extremities has been approved by the U.S. Food and Drug Administration (FDA). The Proteus device, by Angioslide, can be used in the femoral, iliac, iliofemoral, popliteal, tibial, peroneal and profunda arteries.
“One of the most devastating complications arising from peripheral transluminal angioplasty is embolic material that escapes to a point that is anatomically distant from the location of the embolism,” said Gunnar Tepe, M.D., head of diagnostic and interventional radiology at the Academic Hospital of Rosenheim (Rosenheim, Germany). “Such complications can lead to a spectrum of consequences up to an including the loss of limb or life.”
Filter-based systems of embolic protection have several advantages and have been the primary mechanism for capturing embolic debris. However, limitations include the ability to deliver the filter, the added cost, lack of FDA clearance for infrainguinal use and filter-caused arterial trauma.
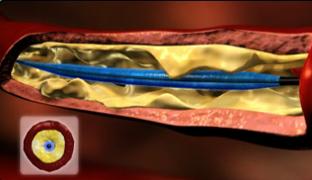
The device is used as an angioplasty balloon, with a technical success rate of 97 percent to 99 percent, and a pooled clinically significant vessel dissection rate of less than 5 percent. Porcine models have shown that there is no increased vessel injury from the inrolling mechanism of the balloon.
It combines the ability to perform angioplasty while also capturing debris that could potentially embolize during the procedure. Initially, the device functions as a normal angioplasty balloon. After angioplasty, the balloon is deflated to 2 atm., when arterial flow remains occluded. The balloon is then infolded upon itself, essentially sucking up potential embolic debris.
For more information: www.angioslide.com


 April 25, 2023
April 25, 2023 









