June 20, 2013 — Biosensors International Group Ltd. has entered into a licensing agreement with Eurocor GmbH, a group company of Opto Circuits (India) Ltd. for their drug eluting balloon (DEB) technology and related intellectual property (IP) rights in relation to the treatment of both coronary and peripheral artery disease. As a first step in this process, an original equipment manufacturer (OEM) arrangement is being implemented, whereby Biosensors will market and sell, under its own brand, DEBs manufactured by Eurocor.
Both agreements will involve three Biosensors-branded DEBs: BioStream; BioPath 014; and BioPath 035. All three devices are drug-eluting balloon dilatation catheters designed for percutaneous transluminal angioplasty (PTA): BioStream has been developed for use in coronary arteries; the two BioPath DEBs have been optimized for the treatment of patients with peripheral arterial disease. BioPath 035 is intended for peripheral intervention above the knee and BioPath 014 for infrapopliteal intervention below the knee.
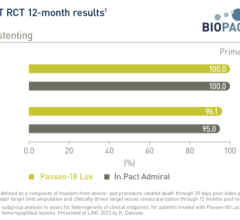
All three DEBs feature Eurocor’s proprietary coating technology delivering paclitaxel, an antirestenotic drug proven in DEB applications. The coating technology has been proven in several clinical studies performed by Eurocor, including the global Valentine I and II registries. The proprietary coating employed in these DEB products is capable of delivering the antirestenotic drug consistently during very brief inflation times, while also minimizing washout of the drug during delivery and placement of the DEB.
“We are delighted to have acquired a new range of leading DEBs as a result of this licensing agreement with Eurocor,” commented Jeffrey B. Jump, president of Biosensors’ Cardiovascular Division. “These innovative devices will complement our existing portfolio of cardiac stents, and facilitate our entry into the peripheral vascular disease market.”
Following CE mark approval currently anticipated within the next six weeks, BioStream, BioPath 014 and BioPath 035 will be launched in major European markets and selected regions in the Middle East, Africa and Asia.
“This agreement is further validation for Eurocor’s DEB products. There is considerable scope for global expansion within this rapidly-growing sector of the interventional devices market,” added Dr. Antonino Laudani, COO of Eurocor GmbH. “We are looking forward to working closely with Biosensors to further the process of establishing Eurocor as one of the leading global providers of innovative DEB products”.
"This arrangement with Eurocor is a positive development in the broadening of our cardiovascular business,” concluded Jack Wang, CEO of Biosensors. “It also marks the first step in our expansion into the peripheral business."
For more infoirmatiuon: www.biosensors.com

 August 22, 2025
August 22, 2025