April 20, 2016 — Boston Scientific Corp. announced a U.S. Food and Drug Administration (FDA) Class 1 recall of the Fetch 2 Aspiration Catheter because the catheter shaft may break at various points along the device, before or during procedures. If breakage occurs while the device is in a patient, pieces of the catheter may block blood supply to the heart or blood vessels. This could result in the need for additional medical procedures, patient injury, or death.
The company initiated the recall on March 22, affecting 17,455 devices nationwide (including Washington, D.C. and the Virgin Islands). The affected devices were manufactured between June 11, 2014 and Feb. 19, 2016, and distributed between June 24, 2014 and March 11, 2016.
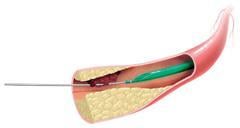
The Fetch 2 Aspiration Catheter is intended to remove small blood clots from peripheral veins and coronary arteries to restore blood flow to the heart.
Customers are advised to discontinue use of all affected products immediately and return unused products to Boston Scientific.
For more information: www.bostonscientific.com, www.fda.gov


 November 14, 2025
November 14, 2025 









