February 24, 2016 — A single-center study noted that treatment with Cardiac Dimensions’ Carillon Mitral Contour System resulted in significant improvement of functional mitral regurgitation (FMR), a condition that can increase the symptoms of heart failure. The study was recently published in the Journal of Invasive Cardiology.
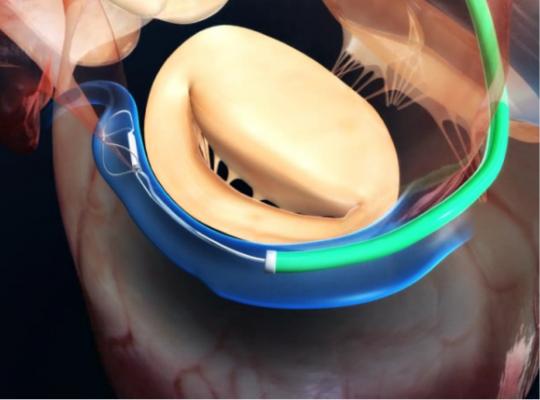
The Carillon device is a percutaneous mitral annuloplasty treatment option that can be deployed rapidly and safely, utilizing standard interventional techniques. Through multiple clinical trials, the device has demonstrated compelling efficacy, significantly improving patients’ symptoms, mitral regurgitation and quality of life.
Researchers of the study noted that percutaneous treatment for FMR is clinically valuable due to the widespread prevalence of the problem and the lack of alternative available therapies.
“In this ‘real-life’ setting, we observed a marked reduction in FMR and continued improvement in the condition over time, with the majority of patients experiencing clinical success similar to the results of the TITAN Trial,” said Norbert Klein, M.D. University Hospital Leipzig, Department of Cardiology and Angiology and lead author of the study. “In addition, we experienced short procedure times, ease of implantation and compatibility with other cardiac therapies.”
Data were collected from a prospective, single-center, non-randomized observational analysis of 17 consecutive patients with moderate to severe FMR, despite stable optimal medical drug treatment. Patients were selected for percutaneous rather than surgical approach due to high surgical risk as assessed by EuroSCORE II and STS score.
The implantable device consists of a distal anchor and a proximal anchor connected by a shaping ribbon and utilizes the heart’s venous anatomy to reshape the mitral annulus. This approach allows for reduction of the dilated annulus, addressing a root cause of FMR.
The device is approved for use in Europe. It is not approved for use in the United States.
For more information: www.cardiacdimensions.com


 December 24, 2025
December 24, 2025 









