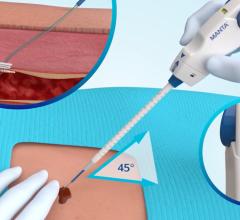
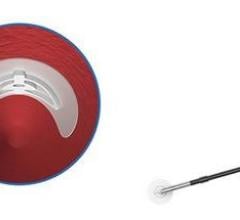
September 20, 2007 - Cook Medical and Cardica Inc. announced that they have expanded their original agreement regarding the development of vascular closure devices, in which Cook Medical will pay Cardica up to $750,000 for the development of an additional product for the closure device product line.
Similar to the original agreement under which Cardica developed the Cook Vascular Closure Device (CVCD), under this amendment Cardica is responsible for product design and pre-clinical development and is entitled to receive royalties on any future worldwide sales by Cook. Cook is responsible for clinical development and regulatory approval, and has exclusive commercialization rights.
According to the manufacturers, advantages of the closure devices developed by Cardica and Cook include a simple user interface, the ability to place it through the same introducer sheath used for the interventional procedure for greater convenience and speed, scalability and lower cost of goods. The companies plan to launch the first product of the Cook Vascular Closure Device line in Europe in the next six months.
For more information: www.cookmedical.com and www.cardica.com

 October 07, 2025
October 07, 2025