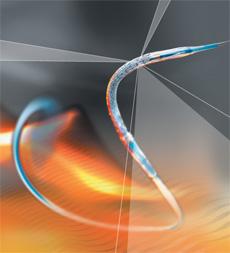
September 1, 2010 - A new zotarolimus-eluting coronary stent using a biocompatible polymer gained CE mark and is being launched in Europe this week. The Medtronic Resolute Integrity Stent System features a novel drug-eluting coronary stent with good deliverability, the ability of the device to traverse the patient’s vasculature and reach the blockage in the heart artery.
Based on the engineering advance of continuous sinusoid technology, the Resolute has been shown in bench testing and in blind in-vivo physician assessment studies to be significantly more deliverable than the current market-leading alternatives.
“Medtronic stents built on continuous sinusoid technology are noticeably more deliverable, giving me greater confidence that I can reach the target lesion, even in patients with especially tortuous anatomy or narrow vessels,” said Dr. Neal Uren, consultant cardiologist at Lothian University Hospitals NHS Trust, Royal Infirmary of Edinburgh, U.K., and honorary senior lecturer at the University of Edinburgh in Scotland. “It’s a significant innovation for the clinical practice of interventional cardiology that sets a new gold-standard for deliverability.”
Coupled with the MicroTrac delivery system, continuous sinusoid technology improves deliverability without compromising other important stent design characteristics, like radial strength. It also improves the stent’s conformability, the ability of the stent to conform to the natural shape of the vessel and maintain apposition to the vessel wall after inflation with the balloon catheter.
The Resolute Integrity drug-eluting stent (DES) combines continuous sinusoid technology with two key elements of the original Resolute DES: the cytostatic antiproliferative drug zotarolimus and the highly biocompatible BioLinx polymer.
For more information: www.medtronic.com


 November 24, 2025
November 24, 2025 









