November 1, 2017 — Results from the prospective, randomized, multicenter CULPRIT-SHOCK trial found an initial strategy of culprit lesion-only percutaneous coronary intervention (PCI) reduces the composite of 30-day mortality and/or severe renal failure in patients with multivessel disease and cardiogenic shock complicating acute myocardial infarction.
Findings were reported at the 29th annual Transcatheter Cardiovascular Therapeutics (TCT) scientific symposium sponsored by the Cardiovascular Research Foundation (CRF), Oct. 29-Nov. 2. The study was also simultaneously published in The New England Journal of Medicine.
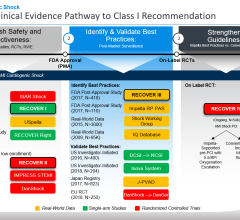
In acute myocardial infarction complicated by cardiogenic shock, early revascularization of the culprit artery by PCI improves outcomes. However, most cardiogenic shock patients present with multivessel disease, and the best revascularization strategy for non-culprit arteries is unknown, with some guidelines favoring more complete revascularization in these patients.
The CULPRIT-SHOCK clinical trial randomized 706 patients with multivessel disease and cardiogenic shock to either culprit lesion-only PCI, with the option of staged revascularization, or immediate multivessel PCI. The primary efficacy endpoint was 30-day mortality or severe renal failure requiring renal replacement therapy. Safety endpoints included assessment of bleeding and stroke. Approximately half of enrolled patients had been resuscitated prior to randomization, 62 percent presented with ST-segment elevated myocardial infarction (STEMI), and 28 percent received some form of hemodynamic support.
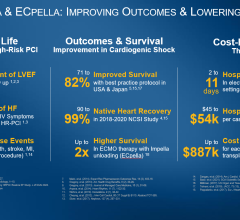
The rate of the primary composite endpoint was significantly lower in patients assigned to culprit lesion-only PCI compared to immediate multivessel PCI (45.9 percent vs. 55.4 percent; RR 0.83; 95% CI 0.71-0.96; P=0.01). There was a significant difference between study groups with respect to all-cause mortality (43.3% percent vs. 51.5 percent; RR 0.84; 95% CI 0.72-0.98, P=0.03), while the difference in the rates of renal replacement therapy was not statistically significant (11.6 percent vs. 16.4 percent, RR 0.71; 95% CI 0.49-1.03, P=0.07). There were no significant differences in the time to hemodynamic stabilization, length of intensive care unit stay, or requirement for and duration of catecholamine therapy.
“Cardiogenic shock during myocardial infarction is a relatively rare, but extremely dangerous condition, in which the heart is unable to pump enough blood to meet the body’s needs,” said Holger Thiele, M.D., director of the Heart Center Leipzig - University Hospital in Leipzig, Germany. “Not only is CULPRIT-SHOCK the largest randomized trial in cardiogenic shock, it is also the first randomized trial to assess a strategy of multivessel PCI versus culprit vessel-only PCI with the option of staged revascularization in this patient population. Based on these 30-day results, culprit vessel only PCI reduces mortality or severe renal failure.”
The CULPRIT-SHOCK trial was funded by the European Union, German Heart Research Foundation and the German Cardiac Society. Thiele reported no other conflict of interest.
For more information: www.tctconference.com
Related Content
TCT Announces 2017 Late-breaking Clinical Trial Presentations
Detroit Cardiogenic Shock Initiative Goes National at TCT 2017
VIDEO: Cardiogenic Shock Supported With Impella Shows Good Outcomes


 April 28, 2023
April 28, 2023