August 28, 2007 - Human embryonic stem cell (hESC)-derived cardiomyocytes improve heart function when transplanted after myocardial infarction, according to a study published in Nature Biotechnology.
The study, led by researchers at Geron Corp. in collaboration with Charles Murry, M.D., Ph.D., and Michael Laflamme, M.D., Ph.D., at the University of Washington, have demonstrated the potential clinical utility of regenerating damaged heart muscle by injecting hESC-derived cardiomyocytes directly into the site of the infarct.
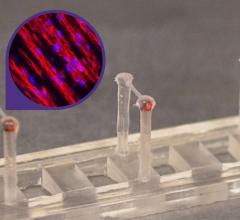
The study describes the feeder- and serum-free, scalable production of hESC-derived cardiomyocytes, their survival in the infarct zone of rats when transplanted four days after infarction and echocardiographic and MRI evidence of significant improvement in cardiac structure and contractile function. The showed that cardiomyocytes from human cardiac cells survived after injection into an infarcted ventricle and to produce significant improvement in heart function. hESCs are the only cell type shown definitively to form cardiomyocytes."
In addition, the research demonstrates the effectiveness of a scalable production system that enables Geron to manufacture the cardiomyocytes for use in ongoing large animal studies and, ultimately, testing in humans.
“We’re developing our cardiomyocyte product, GRNCM1, to address the large unmet need in heart failure,” said Thomas B. Okarma, Ph.D., M.D., Geron's president and chief executive officer. “We expect GRNCM1 to be our second hESC-derived cell type to enter clinical development.”
For more information: www.nature.com and www.geron.com


 November 19, 2021
November 19, 2021