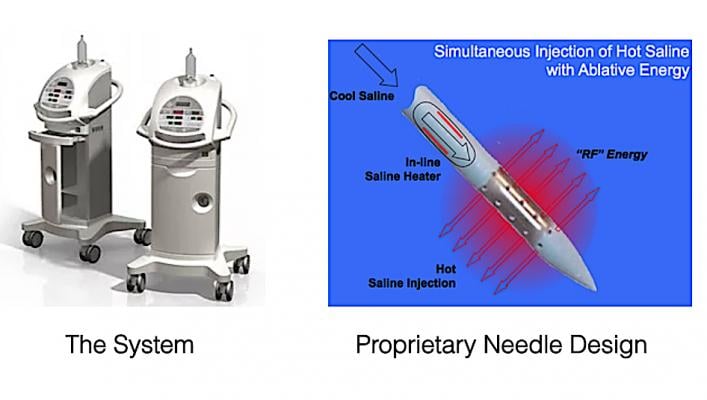
June 16, 2020 – The Montreal Heart Institute (MHI) and Thermedical, a developer of thermal-ablation systems to treat ventricular arrhythmias, announced the first ablation performed using the Durablate retractable needle catheter and degassed saline, which together eliminate over 98 percent of the air bubbles that can lead to stroke.
Health Canada and the U.S. Food and Drug Administration (FDA) approved Thermedical’s degassed saline for use with its Saline Enhanced Radiofrequency (SERF) Ablation system and Durablate catheter, currently being studied for the treatment of ventricular tachycardia (VT), a leading cause of sudden cardiac death worldwide.
“Although ventricular arrhythmias cannot always be completely cured, the new SERF irrigated needle ablation technique with degassed saline solution represents a great advance for patients with extensive diseases, and for whom the next treatment options would be a heart transplant or mechanical heart,” said Katia Dyrda, M.D., associate professor of medicine, electrophysiology training program director, Montreal Heart Institute.
“Moreover, this innovative procedure has the power to greatly improve the quality of life for patients, and I am extremely happy to work in a hospital that makes breakthrough treatments like this available to patients,” continued Dyrda.
SERF ablation with the Durablate catheter is an effective treatment option for patients with ventricular arrhythmias that are resistant to antiarrhythmic drugs or standard ablation procedures. The reason is that Thermedical’s SERF ablation using degassed saline can reach abnormal muscle fibers deep in the heart wall where standard ablation cannot reach and where life-threatening arrhythmias are often located. In addition, Thermedical’s ablation system with degassed saline reduces air bubble volume generated during VT ablation by greater than 98 percent. Clinical research suggests that air bubbles may contribute to procedure-related strokes.
Dyrda, who is leading Montreal Heart Institute’s participation in the SERF VT Ablation study, was the second practitioner to perform an ablation using the new technology in Canada, and the first to perform this intervention using degassed saline. To date, 11 patients have benefitted from the SERF ablation procedure at MHI and three were treated with the new degassed saline. In total, there have been 21 patients treated with SERF ablation in Canada, and five treatments also included degassed saline.
“We hope to one day eliminate or reduce VT episodes in patients and, as a result, significantly improve their quality of life,” said Michael Curley, Ph.D., FHRS, co-founder and chief executive officer of Thermedical. “We are extremely encouraged by Dr. Dyrda and her success in performing ablation using our technology, and we are honored that she is the first in the world to perform it at MHI using our new degassed saline.
“Further, gas released during any form of ablation therapy is a clinical concern for potential procedural-related stroke,” Curley said. “We believe that the use of degassed saline can similarly reduce the amount of gas produced during other ablation procedures such as ablation for atrial fibrillation. We will work to demonstrate the clinical utility of degassed saline for all forms of ablation therapy, and we hope to show that it has widespread ability to reduce gas generated during ablations.”
For more information: www.thermedical.com


 February 06, 2026
February 06, 2026 









