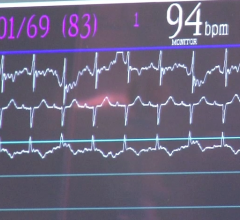
January 25, 2008 - Cheetah Medical Inc. said this week the FDA cleared for marketing the Reliant, a portable cardiac output monitor that provides a noninvasive window to cardiac and hemodynamic function.
The Reliant monitor is powered by Cheetah’s BIOREACTANCE Technology, which has been used effectively with more than 1,000 patients in various clinical settings including hemodynamic monitoring, heart failure, perioperative care, hemodialysis and stress testing.
Reliant uses the NICOM system, which combines traditional impedance technology with BIOREACTANCE technology. Traditional bioimpedance analyzes changes in voltage of electrical currents traversing the patient’s chest, while BIOREACTANCE also analyzes frequency related effects. NICOM’s hybrid analysis helps deal with electrical noise interference, patient movement, respiration and electrode misplacement. As a result, NICOM enables a highly precise, accurate and responsive measurement of cardiac function and hemodynamic status not available with other modalities.
For more information: www.cheetah-medical.com
News | January 24, 2008

 January 13, 2026
January 13, 2026