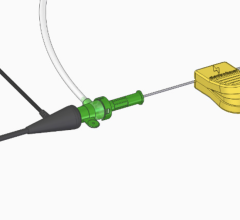
January 31, 2011 – The U.S. Food and Drug Administration (FDA) has approved a system for accessing and delivering diagnostic, embolic and therapeutic materials into the peripheral vasculature. The Renegade HI-FLO Fathom Pre-Loaded System, by Boston Scientific, will be used by interventional radiologists for minimally invasive procedures to treat uterine fibroids and liver cancer.
The system combines the turn-for-turn torque response, flexibility and high visibility of the Fathom-16 Steerable Guidewire with the clinically proven performance of the Renegade HI-FLO Microcatheter, pre-loaded in a single convenient platform. It will be available in eight configurations to suit a broad range of peripheral embolization procedures.
"The excellent deliverability, torque transmission and flow capacity of the Renegade HI-FLO Fathom Pre-Loaded System provides physicians with the performance they need to efficiently access tortuous vessels across many types of interventional oncology procedures," said Jeff Geschwind, M.D., professor of radiology, surgery and oncology, and director of vascular and interventional radiology at the Johns Hopkins University School of Medicine. "Having the Fathom-16 Guidewire pre-loaded in the Renegade HI-FLO Microcatheter will reduce my procedural preparation time and the number of devices that my staff must manage."
Uterine fibroids are non-cancerous growths that develop in the muscular wall of the uterus or cervix, and are estimated to occur in up to 40 percent of women of child-bearing age. Although most uterine fibroids are asymptomatic, some can cause heavy and painful menstrual bleeding, pelvic pressure and frequent urination. Treatment options include medication, hysterectomy, myomectomy (surgical removal of fibroids from the uterus) and uterine artery embolization (UAE). In UAE, a physician uses minimally invasive techniques under local anesthesia to access and occlude both uterine arteries, reducing or eliminating blood flow to the fibroid. Uterine fibroids are the most common benign tumors in women and are the most frequent indication for hysterectomy among pre-menopausal women.
Liver cancer is one of the most commonly diagnosed forms of cancer worldwide, with more than 800,000 patients diagnosed annually. The average life expectancy of many patients with liver cancer is less than one year. Treatment options include surgical resection, chemotherapy, radiation, tumor ablation and several minimally invasive options, including transarterial chemoembolization (TACE) and radioembolization. In the minimally invasive options, a physician will establish endovascular access to the hepatic artery under local anesthesia to deliver one of several acute therapies.
For more information: www.bostonscientific.com


 June 17, 2024
June 17, 2024