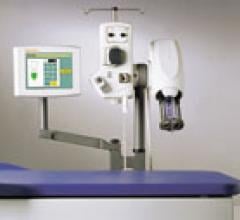
April 17, 2007 — E-Z-EM Inc. has announced it has received 510(k) clearance from the FDA for its EmpowerMR injector system — the company's first product for the magnetic resonance (MR) imaging market.
EmpowerMR has the same easy-to-use interface and robust safety features as the company's other Empower injector line. EmpowerMR also employs several innovative features designed to cope with the problem of electrical interference in the magnetic field of the MR scanner.
Magnetic resonance imaging systems operate by detecting minute variances in the alignment of molecules within certain tissues of the human body. To detect these variances, MR systems create powerful magnetic fields, and require the use of non-magnetic materials and components in the immediate surroundings of the scanner. Electromechanical devices like injector systems can create both electric and magnetic fields that cause electrical interference with the scanner during the imaging process, leading to image distortion or artifacts.
To minimize this problem, EmpowerMR employs a unique, patent-pending hydraulic control system instead of the shielded electrical control components used by most other MR injectors systems.
EmpowerMR has no shielded iron core motors, piezoelectric motors or electrically active motor control circuitry adjacent to the scanner, which significantly minimizes the prospect of electrical interference with the scanner's magnetic field. The device provides for utilization in MR field strengths up to 7 Tesla.
Not battery operated, EmpowerMR links to its electrical supply by a single pass-through cable that does not require special shielding. As there is no motor in the MR suite, EmpowerMR does not generate audible noise. EmpowerMR's hydraulic control also means the system can deliver consistent flow rates, volumetric and pressure performance on demand — features that may help improve productivity in the MR suite.
According to recent data from IMV Limited, a medical technology market analysis firm, there are approximately 6,000 MR scanners in the U.S. and approximately 15,000 worldwide. Approximately 20 percent of MR procedures involve the use of injected contrast.
For more information visit www.ezem.com.


 January 11, 2024
January 11, 2024