December 3, 2010 – The U.S. Food and Drug Administration (FDA) has given conditional approval for an Investigational Device Exception (IDE) application for a system for treating coronary artery disease. The pivotal trial will look at the Tryton Side Branch Stent.
Martin B. Leon, M.D., professor of medicine and director of the Center for Interventional Vascular Therapy at Columbia University Medical Center and founder and chairman emeritus of the Cardiovascular Research Foundation, will serve as principal investigator of the study.
“There is a significant need for alternative solutions for treating bifurcation disease, a persistent and challenging problem for interventional cardiologists that occurs frequently – in about two out of ten cases,” Leon said. “Data from previous studies of the Tryton solution have been highly encouraging, and I look forward to results from this important trial.”
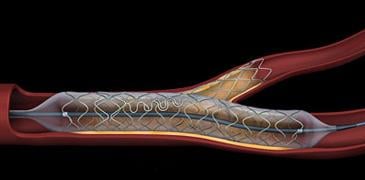
Coronary artery disease often results in the buildup of plaque at the site of a bifurcation, where one artery branches from another. Current approaches often leave the side branch unstented, making it vulnerable to higher rates of restenosis, the renarrowing of the stented vessel following implantation.
The randomized, controlled study will enroll 700 patients at up to 75 centers in North America and Europe.
The Tryton Side Branch Stent System is designed to offer a dedicated strategy for treating atherosclerotic lesions in the side branch at the site of a bifurcation. The cobalt chromium stent is deployed in the side branch artery using a standard single-wire balloon-expandable stent delivery system. A conventional drug-eluting stent is then placed in the main vessel.
The stent system received CE mark approval in Europe and is commercially available in 21 countries throughout Europe and the Middle East. It is not approved in the United States.
For more information: www.trytonmedical.com


 January 05, 2026
January 05, 2026 









