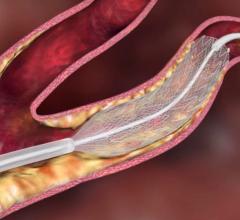
January 27, 2011 – The U.S. Food and Drug Administration (FDA) Circulatory System Devices Panel of the Medical Devices Advisory Committee agreed this week that carotid stenting was safe for patients at standard surgical risk. The panel said there is reasonable assurance that the benefits of the Abbott RX Acculink carotid stent system outweigh its risks (yes: 7, no: 3, abstain: 1), possibly paving the way to expanding the stent’s indicated use.
The committee's recommendation followed a review of data from CREST (Carotid Revascularization Endarterectomy vs. Stenting Trial), the largest prospective study conducted to date comparing stenting to surgery. CREST demonstrated that carotid artery stenting and carotid surgery had similar safety and long-term outcomes for patients with symptomatic and asymptomatic carotid artery disease who were at standard surgical risk. In an analysis of 2,307 standard-risk patients with symptomatic and asymptomatic disease, carotid artery stenting demonstrated noninferiority to surgery for the primary composite endpoint of death, any stroke and myocardial infarction (heart attack) within 30 days of the procedure plus ipsilateral (same side) stroke from 31 to 365 days. The results showed 7.1 percent of stenting patients and 6.6 percent of surgery patients experienced an event, the difference being non-significant.
Currently, RX Acculink is approved for the treatment of symptomatic and asymptomatic patients at high risk of adverse events from carotid endarterectomy (surgery). The FDA will take into account the panel's advice in making its decision on whether to expand the stent’s current indication to include patients at standard surgical risk. The company hopes the FDA will expand the indication later this year.
The Society for Cardiovascular Angiography and Interventions (SCAI) welcomed the panel’s recommendation. SCAI urges the FDA to approve this recommendation, so patients who are candidates for carotid revascularization will have access to a minimally invasive option for treatment of carotid artery disease.
“SCAI urges the FDA to accept the panel’s recommendation, as it would provide patients with a safe, effective, and minimally invasive treatment option for carotid artery disease,” said Christopher J. White, M.D., FSCAI, SCAI president-elect and chairman, department of cardiology and director of the Ochsner Heart and Vascular Institute, Ochsner Medical Center in New Orleans. “This recommendation by the FDA panel to expand the labeled indication for the use of carotid artery stenting would be a step forward in preventing stroke, the third leading cause of death in the United States.”
SCAI supports the recommendation of the FDA panel based on the results of CREST (New England Journal of Medicine, 2010; 363 [1]). Guidelines for secondary stroke prevention recently updated by the American Heart Association and American Stroke Association also include broader recommendations for carotid artery stenting than those currently covered by Medicare. Currently, Medicare and most insurers in the United States cover carotid artery stenting only for symptomatic patients at increased surgical risk and those enrolled in clinical trials. All other patients must pay out of pocket for the procedure, receive medications only, or undergo carotid endarterectomy, where a surgeon makes an incision in the patient's neck to remove the plaque blockage in the carotid artery.
“The FDA panel’s recommendation, supported principally by the data from CREST, reinforces the importance of scientifically rigorous clinical trials that study alternative treatment options for patients. Landmark trials such as CREST provide the evidence base that enables us to offer the right treatment to the right patients at the right time,” said Tyrone Collins, M.D., FSCAI, chair of SCAI’s Carotid Stenting and Neurovascular Interventions Committee and director of interventional cardiology at the Ochsner Heart and Vascular Institute, Ochsner Medical Center in New Orleans. “We are hopeful more patients who may be at risk of stroke will have the full range of treatment options available to them.”
For more information: www.abbott.com, www.scai.org

 June 26, 2023
June 26, 2023