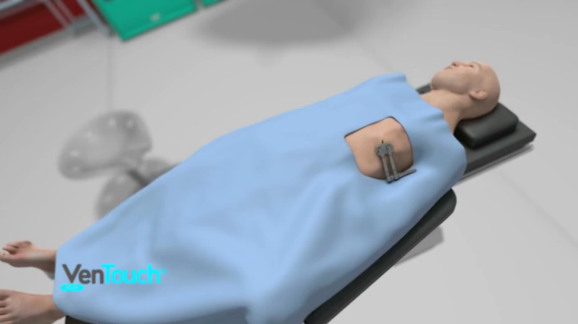
August 15, 2014 — Mardil Medical announced earlier this year the first-in-human implants using the VenTouch ventricular reshaping device performed by a surgical team at the Institut Jantung Negara (IJN) National Heart Institute in Kuala Lumpur, Malaysia. This novel, investigational device was implanted in two patients with severe functional mitral valve regurgitation (FMR), which was reduced to only trace amounts after implantation. The patients are part of a multicenter, global clinical study.
Jeswant Dillon, M.D., clinical director of adult surgery, and Mohd Azhari Yakub, M.D., chief cardiothoracic surgeon, performed the implantations at IJN. The first patient presented several challenges due to severe FMR combined with significant heart dilation and poor left ventricular function.
“After implantation of the VenTouch system’s therapy device, the patient’s mitral valve regurgitation was reduced to trace amounts and the patient was moved out of the intensive care unit the following day,” said Dillon. “Our heart team was fascinated with the ease of placement, the immediate performance of the therapy device and the rapid recovery of the very sick patients.”
The procedures were also attended and supported by William Cohn, M.D., FACS, FACCP, FAHA, director of minimally invasive cardiac surgery at the Texas Heart Institute in Houston; Eugene Grossi, M.D., director of cardiac research at New York University Medical Center; and Vivek Rao, M.D., Ph.D., MSc, FRCSC, chief of cardiac surgery at Toronto General Hospital.
“The VenTouch system is a promising technology that addresses both the annular and muscular abnormalities associated with FMR,” said Cohn. “I was extremely impressed with the marked reduction in regurgitation we were able to demonstrate after a quick, off-pump procedure through a small incision.”
FMR occurs when the left ventricle of the heart is enlarged and the mitral valve no longer closes properly. With each heartbeat, blood leaks backwards into lungs, creating debilitating symptoms. If left untreated, FMR overloads the heart and can lead to or accelerate heart failure. The current treatment for FMR is repairing the mitral valve, an open-heart surgical procedure on a bypass pump, which can be difficult to perform and risky for sicker patients. Even after successful repair surgery, FMR can reoccur because the ventricle often continues to get larger.
"Having now successfully treated patients with the VenTouch system, Mardil has taken an important step towards our goal of introducing this ground breaking therapy and giving FMR patients a promising and significantly less invasive treatment option,” said Jim Buck, president and CEO of Mardil Medical.
The VenTouch system is the only device that treats the root cause of FMR – the enlarged, injured left ventricle. The system’s delivery tool deploys the therapy device through a small incision between the ribs and guides it over the beating heart. The therapy device is a biomaterial sleeve fitted with an inflatable, adjustable fluid chamber which applies prescriptive pressure to a targeted location in order to realign valve leaflets, stopping the leaking by improving the shape and reducing the stress of the enlarged heart. If any further changes in the left ventricle occur after implantation, the VenTouch can be adjusted using a remote port.
For more information: www.mardil.com


 December 24, 2025
December 24, 2025 









