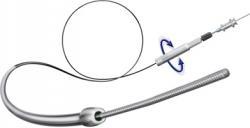
August 5, 2011 — BridgePoint Medical Inc., a Minnesota-based medical device company, has enrolled its first patient in the Peripheral Facilitated Antegrade Steering Technique in Chronic Total Occlusions (PFAST-CTOs) study.
The company received approval from the U.S. Food and Drug Administration (FDA) in July 2011 to move forward with the United States-based investigational device exemption (IDE) for the peripheral devices and is now actively enrolling patients. The 10 center, 100 patient study is designed to assess the safety and effectiveness of the BridgePoint devices in chronically occluded arteries located in the lower extremities.
Subhash Banerjee, M.D., of the VA North Texas Health Care System and UT Southwestern Medical Center stated, "Predicated by the success of the BridgePoint coronary CTO system, we are very optimistic about the Peripheral device's performance possibilities and we are looking forward to a robust and successful study." Banerjee is the first physician to enroll a patient in the study.
The BridgePoint peripheral products are similar to the coronary system in concept, but have been specifically designed and adapted for use in peripheral vasculature and will expand the company's reach into the fast growing peripheral market.
Atherosclerotic peripheral artery disease (PAD) is a widely prevalent disorder that affects millions worldwide and is associated with significant morbidity and mortality. A CTO, which represents a complete artery blockage, typically cannot be treated with standard endovascular interventional devices and is often treated with surgery or medical therapy.
Patients with lower extremity CTOs often suffer from poor circulation in the legs, resulting in debilitating leg pain (claudication), limb threatening ischemia, and limb amputation. Various studies have estimated that chronic total occlusions are common and are found in approximately 50 percent of patients who undergo angiography.
For more information: www.bridgepointmedical.com


 January 05, 2026
January 05, 2026 









