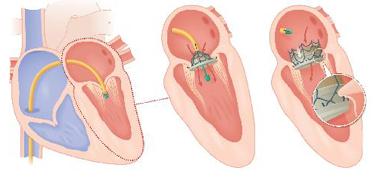
May 21, 2010 – The first successful transcatheter mitral valve implantation (TMVI) for a new mitral valve technology will be highlighted at the EuroPCR 2010 scientific meeting in Paris. A proprietary transcatheter delivery system was used to successfully deliver the valve in the porcine model.
Arshad Quadri, M.D., co-founder, chairman and chief medical officer of Cardiaq Valve Technologies (CVT), will present information about the new self-conforming and self-anchoring valve Tuesday, May 25. The presentation will report six major outcomes of an acute in vivo study of the TMVI system:
• Accurate positioning of the implanted valve relative to the mitral valve annulus
• Secure anchoring of the implanted valve to the mitral anatomy without relying on radial force
• Preservation of the subvalvular apparatus
• Conformance of the implanted valve to the mitral annulus to prevent paravalvular leaks
• Confirmation of a clear, unobstructed left ventricular outflow tract (LVOT)
• The successful delivery and deployment of the implant through an antegrade, transvenous, transseptal, catheter-based approach
Other companies have published results for minimally invasive, transapical approaches to mitral valve replacement. However, CVT believes this is the first time anyone has successfully deployed a mitral valve through a transvenous, transseptal, catheter-based approach.
“Given the enormous unmet clinical need for the overwhelming majority of patients who suffer from mitral regurgitation (MR), we are extremely pleased by this significant milestone,” Quadri said. “The vast majority of MR patients suffer from functional MR and many are too sick to undergo heart valve surgery. In addition, surgical repair is largely ineffective with these patients, as the recurrent rate of MR after repair is about 20 percent. With CVT’s TMVI approach, it appears that easy access along with precise placement may permit a truly interventional or nonsurgical procedure to replace a mitral valve.”
The CVT procedure is designed to be performed in a cardiac catheterization laboratory similar to angioplasty or stenting, resulting in less trauma to the patient and substantial cost-savings to the healthcare system.
For more information: www.cardiaq.com


 December 24, 2025
December 24, 2025 









