January 31, 2014 — W. L. Gore & Associates Inc. (Gore) and AAAneurysm Outreach have partnered to raise awareness and drive screening of individuals at-risk of
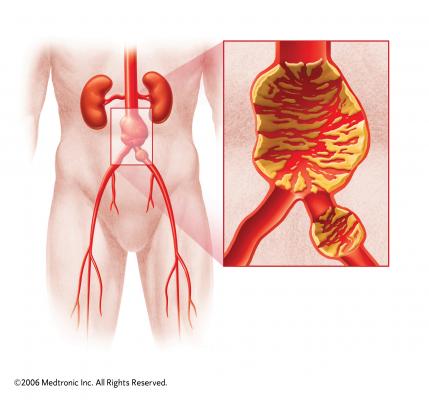
abdominal aortic aneurysm (AAA). The organizations aim to increase programming and education for clinicians and those at risk of AAA, a potentially life-threatening condition caused by a balloon-like of the abdominal aorta.
The partnership and resulting campaign will focus on three key initiatives: awareness, outreach and screening. Raising awareness of AAA through community engagement is a cornerstone of both AAAneurysm Outreach’s approach and Gore’s commitment to patient and clinical groups. AAAneurysm Outreach and Gore will launch an AAA Community Speaker Series where survivors, advocates, providers and at-risk individuals will share experiences and examine issues related to AAA.
Through outreach efforts, Gore and AAAneurysm Outreach will provide materials intended to enhance primary care physicians’ understanding of AAA diagnosis and treatment. The campaign will provide primary care physicians with toolkits to support interaction and communication with vascular surgeons, interventional cardiologists and radiologists within their region.
Finally, the campaign will organize and conduct AAA screenings for those at risk. A key element of this effort will include the AAA Screening Tour, which will empower the patient community to understand risks and facts about AAA. Collaborating with regional healthcare providers, the organizations aim to screen up to 300 individuals at each event.
At-risk individuals include those over the age of 60, those with a history of smoking and those with a family history of AAA.
For more information: www.aoutreach.org, www.gore.com