June 13, 2023 — W. L. Gore & Associates (Gore) announced the initiation of the Gore VBX FORWARD Clinical Study (NCT05811364) to compare the VBX Stent Graft to bare metal stenting for patients with complex iliac occlusive disease.
This prospective, multicenter, randomized controlled trial will include up to 40 sites in the United States, Australia, New Zealand and Europe. An estimated 244 subjects will be randomized 1:1 to the VBX Stent Graft group or the control group (BMS) and will have follow-up visits up to five years from the initial procedure.
The primary endpoint will be primary patency through one year. Secondary endpoints will address technical success, acute procedural success, clinical success, additional patency and target lesion revascularization outcomes and patient improvement metrics. One-year results of the study are expected in 2027, and results are intended to be published and presented at major congresses thereafter.
A cross-specialty physician steering committee, bringing a diverse range of practice experience and perspective, has been assembled to provide joint leadership with Gore on the design and execution of this study.
"There is limited randomized long-term head-to-head outcomes data available to guide stent choice for the treatment of iliac occlusive disease. The VBX FORWARD Study represents an opportunity to positively impact practice guidelines in this area. I look forward to seeing and sharing the data on the full range of clinical scenarios where the VBX Stent Graft may offer an advantage over bare metal stents," said Melissa Kirkwood, M.D., Professor and Chief of Vascular Surgery, University of Texas Southwestern Medical Center, Dallas, Texas and VBX FORWARD Study Steering Committee Member.
"Iliac occlusive disease can be challenging to treat, with tortuous anatomy, severe stenosis and calcified occlusions presenting treatment risks such as perforation and rupture. Covered stents are an integral tool for decreasing the risk of complications in these complex cases and delivering durable outcomes, and the VBX FORWARD Study is thoughtfully designed to further explore those advantages," said Prof. Dr. Michel M.P.J. Reijnen, Vascular Surgeon, Rijnstate Arnhem, The Netherlands and VBX FORWARD Study Steering Committee Member.
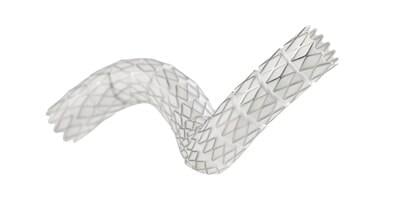
The VBX Stent Graft offers precise delivery and supports positive outcomes in complex aortoiliac applications.1 Recently published five-year long-term follow-up aortoiliac occlusive disease (AIOD) clinical data underscores the robustness and durability of the device.1 The device was developed utilizing the small diameter, ePTFE stent graft technology from the GORE VIABAHN Endoprosthesis. The VBX Stent Graft is available in a range of diameters from 5 to 11 mm and lengths of 15, 19, 29, 39, 59 and 79 mm, currently the longest balloon expandable stent graft available2-7, to cover a variety of occlusive disease treatment needs. The VBX Stent Graft also offers the largest range of diameter adjustability in a single device.2-7
Additional information about the VBX FORWARD Study is available at: https://clinicaltrials.gov/ct2/show/NCT05811364
The VBX FORWARD Study is part of Gore's dedication to research and demonstrating long-term device performance to inform and improve clinical practice and patient outcomes.
"Long-term durability and patient outcomes are the cornerstone of research, development and data generation initiatives across the Gore Medical Products Division. The VBX FORWARD Study is an important step in our continuing efforts to raise the bar for endovascular treatment outcomes that can positively impact patient lives. We hope the results drive much-needed clarity in the iliac occlusive disease treatment algorithm and associated practice guideline recommendations," said Jill Paine, Peripheral Business Leader for Gore's Medical Products Division.
For more information about the VBX Stent Graft, visit: goremedical.com/products/vbx.
Gore engineers medical devices that treat a range of cardiovascular and other health conditions. With more than 55 million medical devices implanted over the course of more than 45 years, Gore builds on its legacy of improving patient out–comes through research, education and quality initiatives. Product performance, ease of use and quality of service provide sustainable cost savings for physicians, hospitals and insurers. Gore is joined in service with clinicians and through this collaboration we are improving lives.
For more information, visit goremedical.com.
References:
- Holden A, Takele E, Hill A, et al. Long-term follow-up of subjects with iliac occlusive disease treated with the Viabahn VBX Balloon-Expandable Endoprosthesis. Journal of Endovascular Therapy. In press.
- GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis [Instructions for Use]. Flagstaff, AZ: W. L. Gore & Associates, Inc; 2019. MD172914.
- LIFESTREAM® Balloon Expandable Vascular Covered Stent [Instructions for Use]. Tempe, AZ: Bard Peripheral Vascular, Inc; 2019. BAW1345700 Rev. 5 06/19.
- iCast covered stent system [Instructions for Use]. Merrimack, NH: Atrium Medical Corporation; 2023. AW009603-EN Rev 11.
- BeGraft Peripheral Stent Graft System [Instructions for Use]. Hechingen, Germany: Bentley InnoMed GmbH.
- BeGraft Peripheral Plus Stent Graft System [Instructions for Use]. Hechingen, Germany: Bentley InnoMed GmbH.
- Advanta V12 Covered Stent [Instruction for Use]. Merrimack, NH; Getinge AB.


 April 26, 2023
April 26, 2023 









