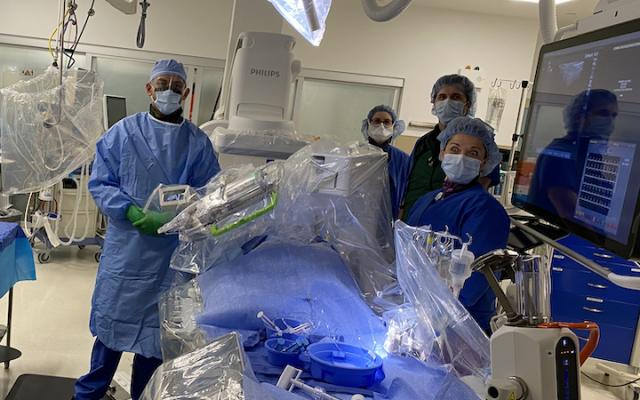
Christopher Baker, M.D., and his team perpare to perform their first robotically navigated carotid artery stenting procedure at Hoag Memorial Hospital Presbyterian. The Corindus Corpath robotic system can be seen over the patient on the table.
May 28, 2020 — Hoag Memorial Hospital Presbyterian recently became the first hospital on the West Coast to perform an innovative robotic interventional procedure to stent a blockage in the carotid artery and improving blood flow to the brain.
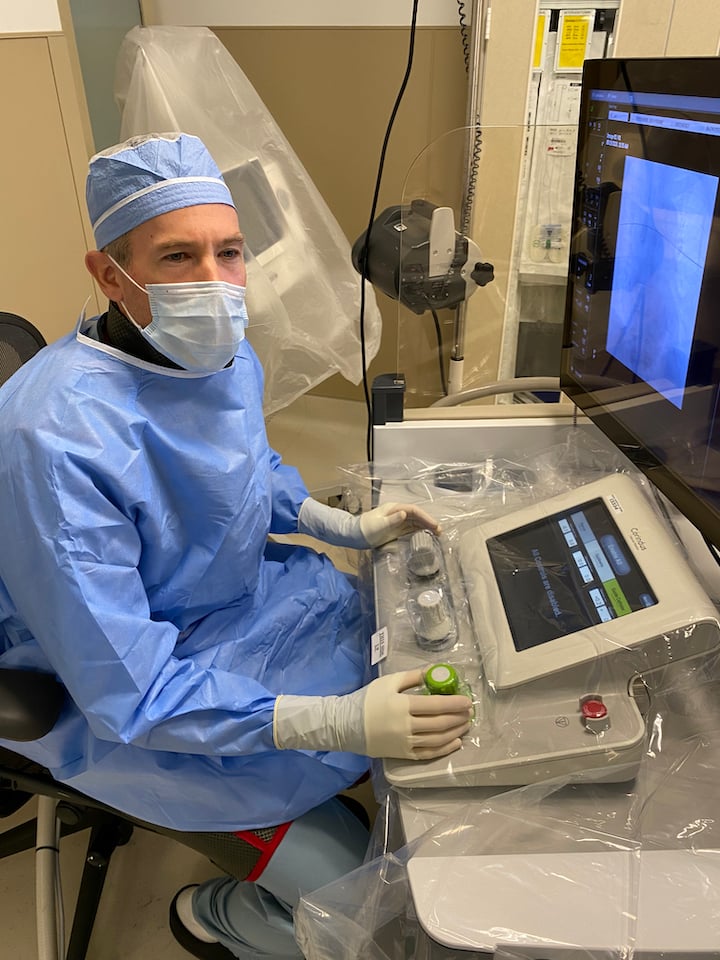 Christopher Baker, M.D., director of interventional neuroradiology at the Pickup Family Neurosciences Institute at Hoag, implanted the stent using a new robotic tool, the CorPath GRX from Corindus, a Siemens Healthineers company. The robot helps physicians guide a wire, stent and balloon through a small incision in the patient’s arm or leg, and up into the patient’s neck artery to place the stent precisely at the site of blockage. This exact placement of the stent also allows less exposure to radiation for both the patient and the physician.
Christopher Baker, M.D., director of interventional neuroradiology at the Pickup Family Neurosciences Institute at Hoag, implanted the stent using a new robotic tool, the CorPath GRX from Corindus, a Siemens Healthineers company. The robot helps physicians guide a wire, stent and balloon through a small incision in the patient’s arm or leg, and up into the patient’s neck artery to place the stent precisely at the site of blockage. This exact placement of the stent also allows less exposure to radiation for both the patient and the physician.
“CorPath is remarkable because I am able to perform a high precision, complex surgery while not being next to the patient,” Baker said. “The use of this technology means a safer, more precise surgery for the patient.”
Thanks to donor support, Hoag’s Pickup Family Neurosciences Institute is the first site on the West Coast to perform robotic-assisted carotid stenting. Currently, robots are only FDA cleared for use in certain general surgery procedures and in peripheral vascular and interventional cardiology procedures. Baker said the tool will likely also prove invaluable in neuro-endovascular procedures. “Carotid stenting has been around for many years, but the really exciting part is the new ability to improve patient safety through precise control of the micro-tools we use in the arteries supplying the patient’s brain.”
As a founding member of Orange County’s designated Comprehensive Stroke Receiving Centers, Hoag’s Neurosciences team helped pioneer many of the advanced processes and specialized methods to optimize outcomes for stroke patients including prevention and reversal of acute stroke.
Hoag has also been designated a Center of Excellence in Robotic Surgery (COERS) by the Surgical Review Corp., which recognizes hospitals and surgeons who demonstrate an exceptional commitment and ability to consistently deliver safe, effective, evidence-based care through robotic surgery.
“We are excited to be one of the first to offer this innovative robotic tool to help treat patients with significant carotid artery stenosis,” Baker said. “One cannot overstate how precision robotic-assisted tools improve outcomes and speed recovery for patients undergoing complex, very delicate surgeries and help us save more lives.”


 November 14, 2025
November 14, 2025 









