May 21, 2018 — The available electrophysiology catheter ablation technologies are not designed for use in the ventricular anatomy, so a new needle ablation catheter from Biosense Webster was tested in a study to assessed the safety.[1] The six-month data from the study was presented at Heart Rhythm 2018, the Heart Rhythm Society’s 39th Annual Scientific Sessions.
All of the patients had failed using a standard approach and then using the needle we were able to get rid of their tachycardia
All 31 patients included in the trial failed standard ablation and medical therapy. However, the needle-based system was able to eliminate their tachycardia.
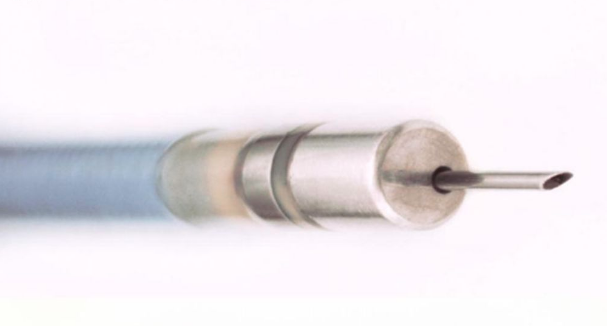
Intramural origin is an important cause of ventricular arrhythmia (VA) ablation failure that we are attempting to address with an irrigated needle ablation catheter. This study assessed the safety and six-month outcome of irrigated needle ablation in patients with sustained monomorphic ventricular tachycardia (VT) and structural heart disease or nonsustained ventricular arrhythmia and left ventricular (LV) dysfunction not responsive to antiarrhythmic drugs and standard catheter ablation.
Patients were enrolled at three centers. At putative target sites selected based on endocardial mapping, the 27 gauge needle was deployed up to 10 mm into the myocardium for recording and pace mapping. Then irrigated with contrast/saline for radiofrequency (RF) ablation with current titrated to a maximum needle temperature of 60 degrees Centigrade.
Irrigated needle ablation was performed in 31 patients at the three centers. Most patients had nonischemic heart disease (NICM) with sustained monomorphic VT (table). A median of 15 RF needle applications per patient were delivered to selected RV/LV septum and free wall sites.
Links to all the Heart Rhythm 2018 Late-breaking Studies
Reference:
#HRS2018 #HRS18


 February 06, 2026
February 06, 2026 









