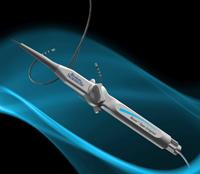
May 3, 2011 – European CE mark approval was granted and the first use announced for the Blazer open-irrigated radiofrequency (RF) ablation catheter. The Boston Scientific catheter is designed to treat a variety of arrhythmias such as atrial fibrillation, atrial flutter, ventricular tachycardia and other supraventricular tachycardias. The product is being launched this quarter in select CE mark countries.
The Blazer integrates a proprietary Total Tip Cooling design with the high-performance Blazer catheter platform. It is intended to consistently cool the entire tip electrode during RF energy delivery. This is achieved through both internal cooling and external washing of the tip electrode, which is designed to reduce coagulum at the proximal edges of the tip.
The first European procedure using the Blazer open-irrigated catheter was performed at the Hospital Cardiologique du Haut–Leveque in Bordeaux–Pessac, France, under the supervision of Michel Haissaguerre, M.D.
"The excellent cooling profile, coupled with the high performance Blazer platform, makes this catheter an important additional option for performing complex ablations," said Sebastien Knecht, M.D., Ph.D., who performed the procedure with Frederic Sacher, M.D. "The tip temperatures were lower than conventional open-irrigated catheters during RF delivery, and the bidirectional catheter performed very well, accessing the pulmonary veins with dependable steering and tip stability."
Affecting more than 4.5 million Europeans, atrial fibrillation is an arrhythmia associated with a rapid rhythm in the upper chambers of the heart. Patients are most often treated with anti-arrhythmic drugs, which can often cause adverse side effects. Cardiac ablation with an RF catheter is increasingly becoming an option for patients who cannot tolerate these medications.
In the U.S., the Blazer open-irrigated catheter is an investigational device, limited by applicable law to investigational use only and not available for sale.
For more information: www.bostonscientific.com


 February 06, 2026
February 06, 2026 









