
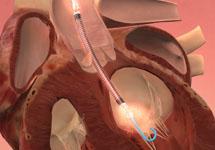
The Impella 2.5 catheter
A 55-year-old woman with a history of heavy alcohol use, hepatitis C induced cirrhosis, hypertension, and chronic renal insufficiency presented at Massachusetts General Hospital in Boston Mass., with nine months of increasing intermittent substernal chest pressure. The pain was inducible by exertion, was nonradiating, and when present associated with shortness of breath, and diaphoresis.
Coronary angiogram showed complex multivessel coronary artery disease. The left main (LM) had a high-grade 90 percent calcified, eccentric stenosis involving the distal vessel segment and extending into the ostium and proximal left anterior descending (70 percent stenosis) and left circumflex (98 percent stenosis) bifurcating coronary arteries.
The patient was evaluated for coronary artery bypass graft, but due to her high surgical risk, poor vein mapping and persistently elevated serum amylase and lipase levels, she was considered a poor candidate. Consensus was made to proceed with high-risk percutaneous coronary intervention (PCI) using the Impella 2.5 cardiac support system.
The Impella is a percutaneously-placed left ventricular support device that provides up to 2.5 liters per minutes of forward flow from the left ventricle (LV) directly into the ascending aorta. The 12 Fr. pump is mounted on the distal end of a 9 Fr. catheter and connected to the Impella Mobile Console.
Transesophageal echocardiography (TEE) was performed prior to the procedure. Findings included a normal LV size and function with evidence of false tendon. There was symmetric LV hypertrophy, mild segmental LV dysfunction involving the inferior wall, a LV true aneurysm at the base of the inferior wall with no evidence of LV thrombus. The estimated ejection fraction was 76 percent.
The left common femoral artery was accessed using a modified Seldinger technique, and upsized to a 12 Fr. sheath to accomodate the Impella catheter. The system was initiated and set at a performance level P8. Heparin was administered to maintain the patient’s activated clotting time above 250. Rotational atherectomy (Rota-blator) was successfully performed from the LM into the LCX artery. Kissing bare metal stents where then subsequently delivered from the LM into the LAD and LCX. Post-stenting kissing balloon angioplasty was then performed with excellent results. The Impella sustained adequate hemodynamic levels throughout the procedure and delivered an average flow of up to 2.1 liters per minute. The mean aortic pressure was maintained around 103 ±3mmHg throughout the procedure, while the mean pulmonary artery pressure averaged 24 to 34mmHg. Immediately following PCI, the Impella was weaned off in stepwise fashion, until it was turned off and removed. Vascular access sheaths were pulled and hemostasis obtained using manual compression. The patient is now chest pain free and attending cardiac rehab.
“This report highlights the valuable use of the 2.5 Impella device in patients with complex high-risk PCI where the alternative surgical revascularization strategy may be otherwise prohibitive or simply too high of risk,” said Roberto J. Cubeddu, M.D., interventional cardiologist at Massachusetts General. “The Impella circulatory support system provides the critical continuous systemic and coronary perfusion time necessary to deliver the preferred utmost complex interventional strategy safely and effectively.”
“Impella allows the interventionalist confidence to be able to use PCI in cases that might otherwise be destined to have a very prolonged and complicated hospital course had they gone to operative therapy,” said Abiomed Medical Director Daniel H. Raess, M.D. “It is notable that in many institutions, some of the most vocal proponents for Impella support are in fact the cardiac surgeons.”


 June 19, 2024
June 19, 2024 









