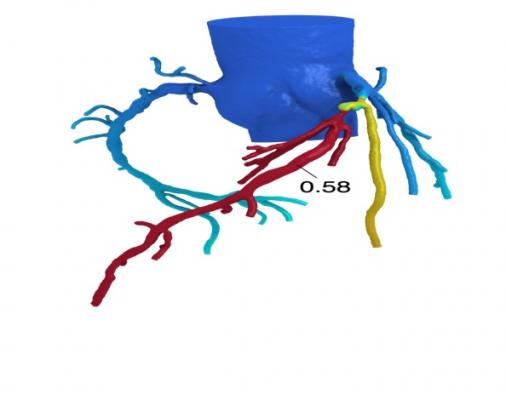
April 5, 2016 — Novel technology developed by HeartFlow Inc. significantly reduces the need for invasive procedures to diagnose patients suspected of having coronary artery disease. The HeartFlow FFR-CT (fractional flow reserve computed tomography) Analysis also leads to a sustained reduction in the cost of care, according to one-year data presented at the American College of Cardiology’s 65th Annual Scientific Session (ACC.16).
The results were unveiled in a presentation on the multicenter, controlled, prospective PLATFORM (Prospective LongitudinAl Trial of FFR-CT: Outcome and Resource Impacts) trial, which compared standard diagnostic strategies to a HeartFlow-guided strategy in 584 patients with stable chest pain. The HeartFlow Analysis is the only non-invasive technology to provide physicians insight into both the extent of a patient’s arterial blockage and the functional impact the blockage has on blood flow.
Key findings of the presentation included that use of a HeartFlow-guided strategy resulted in the cancellation of a planned invasive coronary angiogram (ICA) in 60 percent of patients. After one year, none of the 117 patients who had ICA cancelled had suffered an adverse clinical event. Further, the data showed that use of a HeartFlow-guided strategy resulted in savings to the healthcare system of 33 percent after one year, as compared to patients who received standard care.
“The one-year data affirms use of the HeartFlow Analysis can in many patients safely eliminate the need for invasive catheterizations, and markedly reduce cost of care in patients with suspected coronary artery disease,” said lead investigator Pamela Douglas, M.D., the Ursula Geller Professor at the Duke Clinical Research Institute, Duke University School of Medicine. “This represents a significant advance in the diagnosis and treatment of patients with stable chest pain, who previously may have been sent for unnecessary invasive testing to determine appropriate treatment pathways.”
Studies have shown the need to improve the accuracy of non-invasive tests used to evaluate coronary artery disease. A recent study, which included data from more than 1,100 U.S. hospitals, found that 55 percent of the more than 385,000 patients with suspected coronary artery disease who underwent an ICA had no obstructive coronary disease. In the PLATFORM trial, a HeartFlow-guided strategy reduced the rate of normal or near-normal ICA by more than 80 percent.
The HeartFlow FFR-CT Analysis is a Web-based platform that aids clinicians in diagnosing coronary artery disease, and provides personalized, actionable information to physicians to manage each patient. FFR-CT technology solves millions of complex equations simulating blood flow in the coronary arteries to provide mathematically computed fractional flow reserve values from images derived from non-invasive coronary CT angiography (cCTA). FFR-CT values indicate blood pressure differences around a coronary narrowing to determine whether it is likely to reduce blood flow to the heart.
The HeartFlow FFR-CT Analysis has been evaluated in four large, prospective clinical trials enrolling a total of more than 1,100 patients at major medical centers worldwide. It received CE mark in 2011 and U.S. Food and Drug Administration clearance in November 2014.
For more information: www.heartflow.com


 July 31, 2024
July 31, 2024 









