
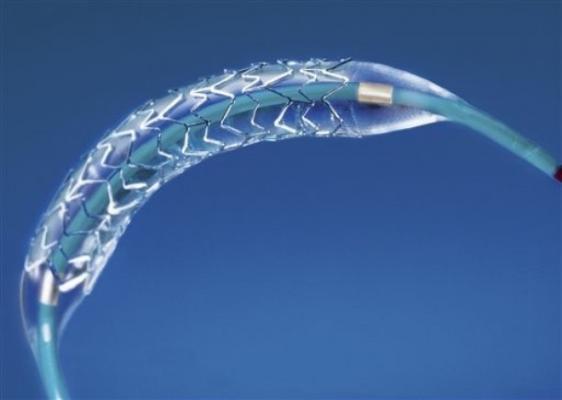
Taxus Element Paclitaxel-Eluting Stent System.
March 15, 2010 — Results from the PERSEUS clinical program demonstrate positive safety and efficacy outcomes in workhorse lesions for a new platinum chromium stent system.
The 12-month results studied Boston Scientific Corp.'s Taxus Element Paclitaxel-Eluting Stent System compared to the Taxus Express Paclitaxel-Eluting Stent System. The results also reported a similar safety profile and statistically superior efficacy outcomes in small vessels for the Element stent compared to a historical control group of patients receiving the Express bare-metal stent.
Analysis of the data was presented at the American College of Cardiology Annual Scientific Sessions this week during a late-breaking trial session by Dean Kereiakes, M.D., medical director, The Christ Hospital Heart and Vascular Center and The Lindner Research Center in Cincinnati, and the principal investigator for the PERSEUS clinical program.
The Element stent is designed specifically for coronary stenting. The novel stent architecture and proprietary platinum chromium alloy combine to offer greater radial strength and flexibility. The stent architecture helps create consistent lesion coverage and drug distribution while improving deliverability, which is enhanced by an advanced catheter delivery system. The higher density alloy provides superior visibility and reduced recoil while permitting thinner struts compared to prior-generation stents.
The PERSEUS clinical program compares the TAXUS Element to prior-generation stents in more than 1,600 patients in two parallel trials at 90 centers worldwide.
Workhorse trial
The PERSEUS Workhorse trial is evaluating the safety and efficacy of the Taxus Element compared to Boston Scientific’s first-generation Taxus Express stent in 1,262 patients with de novo lesions.
The prospective, randomized trial met its primary endpoint of non-inferiority for target lesion Failure at 12 months with rates of 5.6 percent for the Taxus Element Stent and 6.1 percent for the Taxus Express. The secondary endpoint of in-segment percent diameter stenosis at nine months as measured by quantitative coronary angiography (QCA) was also met.
The Workhorse results also demonstrated similar safety for Element as demonstrated by low rates of major adverse cardiac events (MACE) and stent thrombosis. All components of MACE, including cardiac death, myocardial infarction (MI) and target vessel revascularization (TVR) were similar to the TAXUS Express stent control. A numerically lower rate of non-Q-wave MI for the Element stent resulted in lower overall MI (2.2 vs. 2.9 percent). Stent thrombosis rates using the Academic Research Coalition (ARC) definite/probable definition were statistically similar for the Taxus Element and the Taxus Express (0.4 and 0.3 percent).
Small Vessel trial
Results were also presented from the PERSEUS Small Vessel trial, a single-arm study which compares the Taxus Element in 224 patients with small vessels to a matched historical control group of 125 patients treated with the Express bare-metal stent. The trial met its primary endpoint of superiority for in-stent late loss at nine months with unadjusted values of 0.38 mm for the Element stent and 0.80 mm for the Express.
The trial also met its secondary endpoint of superiority for TLF at 12 months, showing a statistically significant reduction with an unadjusted rate of 7.3 percent for Element compared to a prespecified performance goal of 19.5 percent based on historical outcomes for the control stent. The propensity-adjusted MACE rates were significantly lower for the the Element stent compared to the bare-metal control stent (10.5 vs. 30.4 percent), showing a safety benefit for Element thrombosis rates using the ARC definite/probable definition were comparable for the Taxus Element and Express (0.3 vs. 0.6 percent).
“The PERSEUS trials build on the extensive data from the Taxus clinical program and extend the consistent outcomes seen in the Taxus trials to the novel Element stent platform,” said Louis Cannon, M.D., of the Cardiac and Vascular Research Center of Northern Michigan in Petoskey, Mich., and the trial’s co-principal investigator. “With the positive outcomes of the Element stent in workhorse lesions and the superior efficacy data in small vessels, platinum chromium promises to offer significant advantages in acute performance with no compromise to safety.”
Clinical data from the PERSEUS trials will support regulatory approval of the Element Paclitaxel-Eluting Stent System in Europe, the United States and Japan. The company is evaluating its Promus Element Everolimus-Eluting Stent System in the PLATINUM clinical trial, which completed enrollment of 1,531 patients in September 2009 at 133 sites worldwide. PLATINUM is a randomized, controlled, pivotal trial designed to support U.S. and Japanese approval of the Promus Element. Results are expected to be presented in early 2011.
The company received CE mark approval for the Promus Element in October 2009 and expects CE mark approval for the Taxus Element in the second quarter of this year. In the United States, the company expects FDA approval for the Taxus Element in the middle of next year and for the Promus Element in the middle of 2012. In Japan, the company expects approval for the Element in late 2011 or early 2012 and for the Promus Element in the middle of 2012.
Both stent systems are investigational devices in the United States and are limited by applicable law to investigational use only and are not available for sale.
For more information: www.bostonscientific.com


 January 05, 2026
January 05, 2026 









