April 7, 2024 — In patients with severely elevated triglyceride levels at risk for developing acute pancreatitis, the investigational drug plozasiran reduced triglyceride levels by an average of 74% after 24 weeks of use without causing any significant safety concerns, according to research presented at the American College of Cardiology’s Annual Scientific Session.

Daniel Gaudet, MD
“Plozasiran produced significant reductions in triglyceride levels below the threshold associated with elevated risk for pancreatitis, with a favorable safety profile,” said Daniel Gaudet, MD, PhD, professor of medicine at the University of Montreal and the study’s lead author. “These data support the initiation of pivotal studies of plozasiran for the treatment of severe hypertriglyceridemia.”
Triglycerides are blood lipids (fats) that store unused calories and provide energy to the body. An estimated 1 in 5 U.S. adults—and more than 2 in 5 of adults aged 60 years and older—have elevated triglycerides, defined as over 150 mg/dL. High levels of triglycerides and the lipid particles on which they are carried in the blood can contribute to the formation of “plaques” in the arteries that impede blood flow and can lead to heart attacks and strokes. Severe hypertriglyceridemia—defined as triglyceride levels over 500 mg/dL—can also cause pancreatitis, an inflammatory process in the pancreas that can be life threatening.
ApoC3 is a protein produced in liver cells that inhibits the liver’s ability to clear fats such as triglycerides out of the body. Plozasiran works by reducing the production of ApoC3, thereby enabling the liver to increase its clearance of triglycerides and other fats. The SHASTA-2 trial tested the effectiveness and safety of plozasiran as an add-on to existing lipid-lowering treatment in patients with severe hypertriglyceridemia.
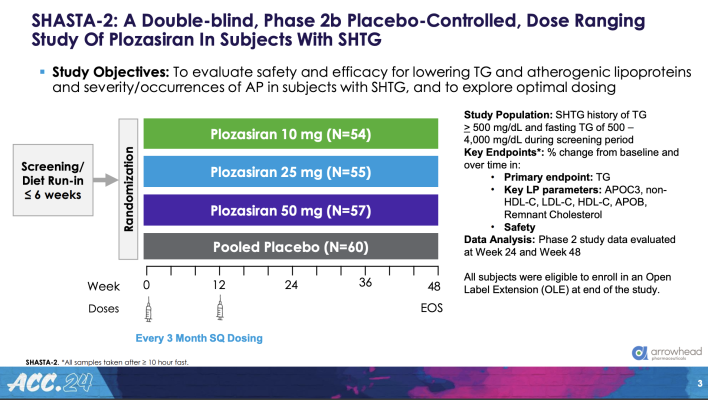
A total of 229 patients (average age 55 years, 78% men and 90% White) were enrolled in the trial in eight countries. Patients’ average triglyceride level at baseline was about 900 mg/dL. Most also had at least three of the following risk factors: elevated risk for or history of cardiovascular disease, diabetes, low HDL (“good”) cholesterol and high body mass index.
Patients were randomly assigned to one of four groups. Three groups received two injections of plozasiran at one of three doses (10 mg, 25 mg or 50 mg); the fourth group received two injections of a placebo. The first injection was given on day one and the second at week 12. The study was double-blinded, meaning that neither the patients nor their clinicians knew who was receiving the drug and who was receiving the placebo until the study was over.
The study’s primary endpoint was the percentage change in fasting triglyceride levels from study entry to 24 weeks. Secondary endpoints included the percentage change in ApoC3 at 24 weeks and every four weeks from baseline through 48 weeks and in fasting triglyceride levels every four weeks through 48 weeks. All patients were followed for 36 weeks after the second dose (for a total of 48 weeks).
At 24 weeks, the average reduction in triglyceride levels in plozasiran-treated patients was 74%, compared with 17% in patients who received the placebo. At 48 weeks the average reduction was 58% in patients who received the highest doses of plozasiran compared with 7% for those in the placebo group. The average reduction in ApoC3 was 78% for plozasiran-treated patients versus 1% for the placebo group at 24 weeks. At 48 weeks, ApoC3 levels were reduced by 48% on average among patients receiving the highest doses of plozasiran, whereas ApoC3 levels increased 4% in the placebo group.
At 24 weeks, over 90% of patients who received the higher doses (25 mg or 50 mg) of plozasiran saw their triglyceride levels fall to below 500 mg/dL, the commonly accepted threshold for an increase in risk for pancreatitis. At 48 weeks, 77% of these patients still had triglyceride levels of less than 500 mg/dL. More than 50% of patients on higher doses achieved triglyceride levels of below 150 mg/dL (i.e., in the normal range) at 24 weeks.
“Significant and durable dose-dependent reductions in ApoC3 and triglycerides persisted through week 48, or 36 weeks after patients received their second dose of plozasiran,” Gaudet said.
Severe hypertriglyceridemia is a challenging condition for which few effective treatments are currently available, he said.
“From the patients’ standpoint, the possibility that in the near future there could be an agent that safely and effectively lowers severely elevated triglyceride levels and reduces or eliminates the risk of developing pancreatitis is extraordinary,” he said.
Limitations of the study are its relatively small size and short duration and a lack of diversity among the enrolled patients, Gaudet said. The planned phase 3 study will be conducted in a patient population that will include more women and more patients from racial and ethnic minorities.
“What we want to know is how sustained is the effect of plozasiran in a larger, more diverse cohort,” he said.
The study was funded by Arrowhead Pharmaceuticals, the manufacturer of plozasiran.
For more information: www.acc.org
Find more ACC24 conference coverage here


 July 31, 2024
July 31, 2024 









