March 29, 2012 — In the second year of an ongoing trial, the Resolute zotarolimus-eluting stent (Medtronic) achieved a low rate of stent thrombosis, cardiac death, target vessel heart attack and target lesion revascularization years, according to research presented at the American College of Cardiology’s (ACC) 61st Annual Scientific Session.
The Resolute U.S. trial reported similar results in year one, examining safety and efficacy outcomes for a zotarolimus-eluting stent that was approved by the U.S. Food and Drug Administration (FDA) in February. The stent includes a new biocompatible polymer that allows for an extended drug release across approximately six months, which has been hypothesized to better prevent restenosis (vessel renarrowing) while maintaining a low rate of stent thrombosis (blood clots).
For the study, 1,402 patients were enrolled at 116 centers in the United States between August 2008 and December 2009. The mean patient age was 64.1 years and 68.3 percent of patients were male; 34.4 percent had diabetes, the highest percentage included in any trials of the zotarolimus-eluting stent to date but a percentage that is characteristic of patients in the U.S. who receive angioplasty. During their angioplasty procedure, each patient received a zotarolimus-eluting stent ranging from 2.25 mm to 4 mm in either one or two lesions. Participants were then followed either with clinical follow-up (n=1242) or angiographic follow-up (n=160) to determine the rates of adverse events.
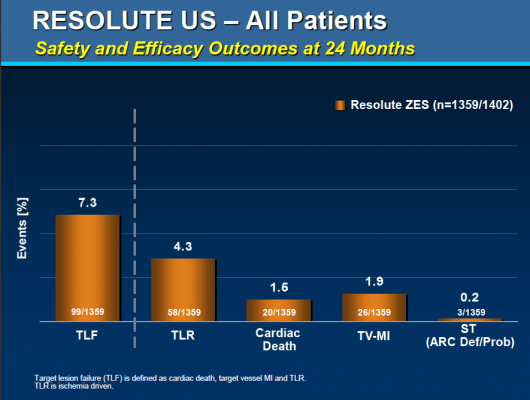
Specifically, the study’s primary endpoint was the rate of target lesion failure, a composite of cardiac death, target-vessel heart attack, and clinically driven target lesion revascularization by angioplasty or surgical methods. The secondary endpoints included the individual rates of cardiac death, clinically driven target lesion revascularization and definite plus probable stent thrombosis.
At the two-year follow-up point, the researchers obtained data from 1,359 of the enrolled participants. Of these, 7.3 percent experienced the primary endpoint of target lesion failure, while 3 percent experienced cardiac death, 1.9 percent experienced target-vessel heart attack and 4.3 percent experienced clinically driven target lesion revascularization; 0.2 percent of patients experienced definite or probable stent thrombosis.
The two-year results were similar to the one-year findings, which included a 4.7 percent rate of target lesion failure and a 0.1 percent of definite or probable stent thrombosis.
According to lead investigator Laura Mauri, M.D., M.Sc., an interventional cardiologist at Brigham & Women’s Hospital and associate professor of medicine at Harvard Medical School, diabetic patients— who generally are more likely to suffer from restenosis or stent thrombosis—did particularly well in the study, with a two-year rate of target lesion failure of 8.2 percent and no stent thrombosis events at two years.
“Within a study population where one-third of the patients were diabetic and 10.7 percent were treated in small vessels, the Resolute U.S. study’s two-year results showed the clinical endpoint of low target lesion failure was achieved with very low stent thrombosis risk,” said Mauri. “These results will greatly facilitate catheter-based treatment of a broader group of patients with symptomatic coronary artery disease.”
The study was funded by Medtronic. Mauri is a consultant to and receives institutional grant support from Medtronic.
For more information: www.acc.org


 January 05, 2026
January 05, 2026 









