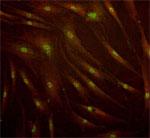
Representative C-Cure immunolabeling of cardiopoietic cells derived from a patient bone marrow sample.
October 16, 2009 – The Clinical Center of Serbia, Institute for Cardiovascular Diseases today said it treated its first patient with a revolutionary cell-based therapy for heart failure.
The patient is participating in an international trial of C-Cure, a second-generation cell therapy developed by Cardio3 BioSciences, a leading Belgian biotechnology company specializing in stem cell-based therapies for the treatment of cardiovascular disease.
C-Cure is produced by taking a patient's own bone marrow cells and, through a proprietary process, differentiating them into “cardiopoietic” cells that can regenerate damaged heart muscle. The cardiopoietic cells are injected into the heart of a patient with heart failure where they are designed to behave identically to those cells lost in heart failure without carrying the risk of rejection, something that has not been achieved with previous cell therapies for this indication.
The trial, a randomized, prospective, multicenter trial, is designed to evaluate the safety and efficacy of C-Cure beyond optimal clinical care in patients with heart failure. Patients will be randomized to C-Cure in addition to optimal standard therapy versus optimal standard therapy alone. The trial will also evaluate socio-economic implications of therapy. The trial is being carried out at various sites in the European Union, and now Serbia.
“C-Cure is a major breakthrough in the field of cardiac regenerative medicine,” said Dr. Jozef Bartunek, coprincipal investigator for the trial. “This clinical trial will be the very first to apply autologous, guided cardiac progenitor cells. This next generation stem cell product could contribute to the physical and functional regeneration of cells in the chronically infarcted heart."
"Heart failure is one of the greatest causes of premature death in the world and we are very pleased to be participating in the trial of a product that could potentially transform the treatment of the condition and the outcome for patients," said Miodrag Ostojic, principal investigator for the trial in the Clinical Center of Serbia, Institute for Cardiovascular Diseases.
Cardio3 BioSciences is a leading Belgian biotechnology company specializing in stem cell-based therapies for the treatment of cardiovascular disease. Mayo Clinic has a financial interest in technology related to this research and may stand to gain from the successful outcome of the research. Mayo Clinic holds equity in Cardio3 BioSciences as a result of intellectual property licensed to the company.
For more information: www.c3bs.com


 January 05, 2026
January 05, 2026 









