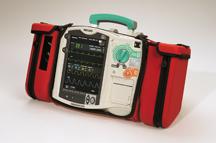
January 29, 2009 - Medusa Medical Technologies Inc.’s flagship electronic patient care reporting software, the Siren ePCR Suite, now has an interface with the Philips HeartStart MRx monitor/defibrillator.
Siren ePCR Suite patient care reporting system reportedly enables paramedics to administer care while recording critical patient data that they transmit in real-time to receiving facilities. Siren ultimately improves data accuracy by downloading data from the HeartStart MRx directly into the tablet PC software, including ECG, vitals, treatments and events. This system helps to support a decrease of discovery-to-treatment times by enabling the patient's care to be evaluated and treatment to be given en route to the hospital.
The Philips HeartStart MRx monitor/defibrillator combines diagnostic patient monitoring, defibrillation and CPR measurement and feedback into a lightweight and intuitive package designed for use by ALS responders and hospital clinicians. For electronic Patient Care Reporting (ePCR) software vendors, Philips offers the HeartStart Data Software Developer Kit (SDK), which allows data from Philips HeartStart monitor/defibrillators and AEDs to flow seamlessly into patient care reports.
Using this new solution, pre-hospital care providers in the field will be able to monitor the patient's current condition, as well as access a patient care database that helps them make more informed decisions and deliver better care to patients. The data from the HeartStart MRx, including patient vitals and waveforms, are recorded in the ePCR, ensuring continuity of care from the ambulance to and through the hospital.
For more information: www.medusamedical.com, www.medical.philips.com


 January 13, 2026
January 13, 2026 









