May 29, 2007 — Frost & Sullivan is launching a new end user study on the state of adoption for atrial fibrillation therapies in the U.S. cardiovascular market. Through more than 150 detailed interviews with electrophysiologists, cardiologists and other cardiovascular professionals, the company aims to elucidate key drivers and obstacles to adoption and utilization of various atrial fibrillation treatments.
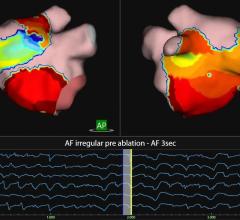
"Currently no ablation system to date has been approved explicitly for use in treating atrial fibrillation," notes Frost & Sullivan Industry Analyst Venkat Rajan. "However, advancements in 3-D mapping, other imaging techniques, and features within the systems themselves are enabling higher success rates for procedures. Thereby expanding confidence among end users, and gathering enough clinical data for possible FDA approval."
While there are numerous medical device therapies that are being used to treat atrial fibrillation, such as minimally invasive surgical ablation, catheter ablation, open heart surgery ablation, pacemakers, anticoagulants, etc., physician awareness and preference are often the primary determinants of the method used.
"There are a vast number of participants in the market from start-ups and entrepreneurial-type companies to nearly every major cardiovascular device manufacturer," explains Rajan. "Each manufacturer seems to be attempting to carve out their own niche in the market, whether it is through energy modality, or navigational capabilities."
"Manufacturers that can better understand the drivers and obstacles of adoption and utilization preferences for atrial fibrillation device therapies can develop and refine their technologies to best serve customer demand," says Rajan. "With effective third party information partners, companies can take steps to ensure their continued success."
For more information, visit www.frost.com.


 September 05, 2025
September 05, 2025