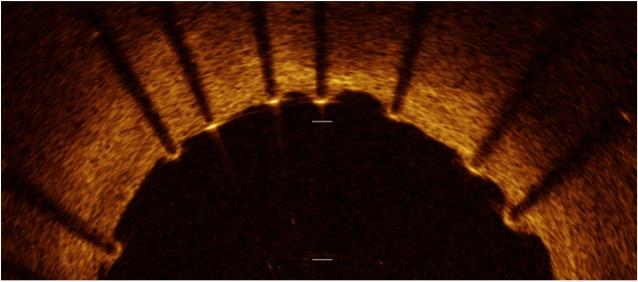
An OCT image showing good stent strut apposition against the vessel wall.
January 13, 2017 — Results from TRANSFORM-OCT, a prospective, randomized trial using optical coherence tomography (OCT) to evaluate strut coverage and neoatherosclerosis (NA) found that bioresorbable polymer metallic drug-eluting stents are comparable to durable polymer-based drug-eluting stents. The study compared the Boston Scientific Synergy bioresorbable polymer everolimus-eluting stent (BP-EES) to the the Medtronic Resolute Integrity durable polymer zoterolimus-eluting stent (DP-ZES).
Findings were reported at the 2016 Transcatheter Cardiovascular Therapeutics (TCT) scientific symposium in November.
To mitigate the risk related to durable polymer (DP), bioabsorbable polymers (BP) that only coat the abluminal side of the stent were developed. This avoids contact with circulating blood. The stent also becomes a bare metal stent after complete absorption of the drug-carrying polymer. It is not known if BP abluminal coatings may counteract in-vivo delayed healing.
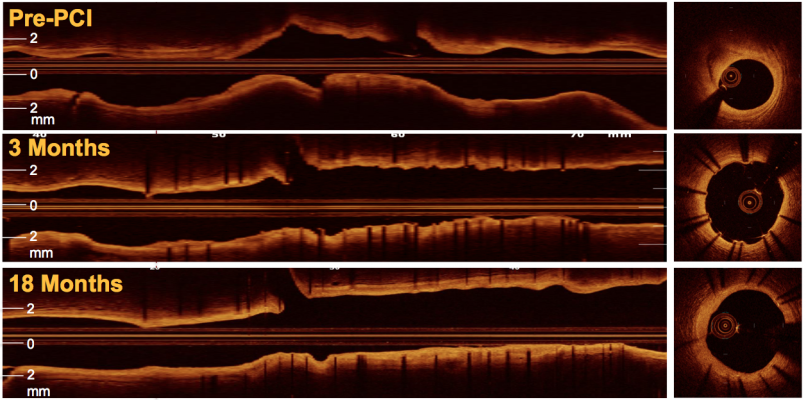
The TRANSFORM-OCT study randomized 90 patients with multivessel disease (1:1) to a BP-EES, Synergy) or a DP zotarolimus-eluting stent (DP-ZES, Resolute Integrity). The primary endpoints were maximum length of consecutive frames with uncovered struts at three months (powered for non-inferiority of BP-EES) and the percentage of patients presenting with frames of NA at 18 months (powered for superiority of BP-EES) as measured by OCT. The three-month median percentage of covered struts was 79.1 (IQR 60.4, 89.8) for BP-EES and 78.4 (IQR 62.1, 87.8) for DP-ZES, P=0.93. The 18-month median percentage of covered struts was 99.4 (IQR 96.6-100) for BP-EES and 98.0 (IQR 94.4-99.8) for DP-ZES, P=0.14. The co-primary endpoint of in-stent NA at 18 months from 98.9% of all eligible patients was 11.6 % for BP-EES versus 15.9% in DP-ZES (P=0.59) with low percentage of frames with NA in both stent types (1.1 ± 3.1 for BP-EES versus 2.5 ± 9.1 for DP-ZES, P=0.33)
“In this head-to-head in-vivo comparison of early and late healing response, the bioresorbable abluminal polymer Synergy everolimus-eluting stent was non-inferior at three-month and similar at 18-month follow-up to the durable conformal polymer Resolute zotarolimus-eluting stent,” said Giulio Guagliumi, M.D. with Ospedale Giovanni XXIII in Bergamo, Italy. “TRANSFORM-OCT adds a novel mechanistic dimension to the assessment of new-generation drug-eluting stents, consolidating the understanding that well designed and biocompatible polymers, regardless of whether they are durable or biodegradable, may favorably impact the long-term vascular response of these stents.”
The TRANSFORM-OCT trial was designed, promoted and implemented by Ospedale Papa Giovanni XXIII, Bergamo (Italy) with unrestricted financial support provided by Boston Scientific International. The company was not involved with any of the study processes, including site selection, data collection, analysis, and drafting of the present abstract. Guagliumi reported consultant agreements with Boston Scientific and St. Jude Medical and receive research grants through the hospital by Abbott Vascular, St Jude Medical and Boston Scientific.
For more information: www.crf.org


 November 14, 2025
November 14, 2025 









