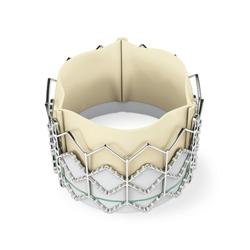
September 24, 2010 - The New England Journal of Medicine has published the results of from a trial looking at the treatment of severe aortic stenosis.
The results from cohort B of the Partner trial, which studied the Edwards Sapien transcatheter heart valve, successfully met the primary endpoints of all-cause mortality and mortality plus repeat hospitalization.
The authors concluded, "in patients with severe aortic stenosis who were not suitable candidates for surgery, TAVI [transcatheter aortic valve implantation], as compared with standard therapy, significantly reduced the rates of death from any cause, the composite end point of death from any cause or repeat hospitalization, and cardiac symptoms, despite the higher incidence of major strokes and major vascular events." The article further states, "on the basis of a rate of death from any cause at one year that was 20 percentage points lower with TAVI than with standard therapy, balloon-expandable TAVI should be the new standard of care for patients with aortic stenosis who are not suitable candidates for surgery."
According to the authors, "aortic stenosis is an insidious disease with a long latency period followed by rapid progression after the appearance of symptoms, resulting in a high rate of death" and concluded that "standard medical therapy... did not alter the natural history of severe aortic stenosis."
This trial studied 358 patients with severe, symptomatic aortic stenosis deemed inoperable for traditional open-heart surgery. Patients were evenly randomized to receive either the Edwards Sapien valve or standard therapy.
The Sapien transcatheter valve is an investigational device in the United States and not yet available commercially in this country.
For more information please visit: www.edwards.com.


 January 05, 2026
January 05, 2026 









