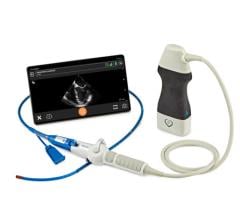
October 12, 2023 — Franklin Mountain Medical announced today that the Dib UltraNav Transseptal Catheter System, which houses a needle and ultrasound in one system for use in atrial transseptal procedures and delivery of catheters, has now been successfully used in 15 human cases, at major cardiology hospitals in Arizona and Minnesota.
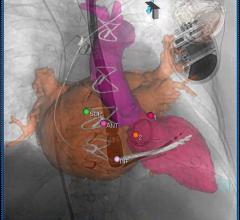
UltraNav improves the safety and accuracy of transseptal procedures by enabling better visualization of the catheter, needle, and needle tip. It facilitates safer and more predictable transport of intracardiac echocardiography (ICE) and other catheters/wires from the right into the left atrium of the heart. Its use also suggests it may reduce the need for Transesophageal Echo (TEE) interventions.
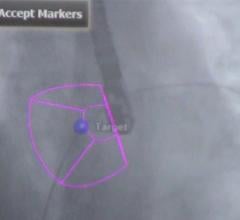
UltraNav replaces the current procedural approach of using a separate needle and ultrasound beam on two different planes, which provides only a partial image of the orientation and depth of the needle and its tip, further disrupted by the cardiac and respiratory motion of the patient. Because UltraNav is a single system that aligns the catheter/needle with the ultrasound beam on the same plane, it provides more precise, continuous, and complete visualization for the clinician. Improved visualization reduces complications and increases catheter alignment during transseptal procedures.
Clinical experience to date indicates that UltraNav may also reduce recovery time in certain procedures because it only requires moderate sedation, not general anesthesia. This has resulted in the elimination of hospital stays and allowed patients to return home in a matter of hours, which reduces scheduling challenges that exist in procedures using TEE and can lead to substantial reductions in overall cost.
"There's been a significant rise in structural transseptal heart procedures, which mandates the need for a safer, more accurate approach," said Dr. Nabil Dib, founder of Dib UltraNav Medical and inventor of the UltraNav Transseptal Catheter System. "Although the current risks may be low, complications can be fatal. Reducing serious complications such as heart perforation and reducing the need for anesthesia with a novel one-catheter system will change the way we approach structural heart interventions, reduce the learning curve for physicians, and expand the treatment to more patients."
Dr. Dib will present "A Double Lumen Catheter to Facilitate Atrial Septal Procedure and the Transseptal Delivery of Catheters: Dib UltraNav Transseptal Catheter System/Catheter-Based Treatment of Congenital Heart Disease: ASD, PFO, and RVOT Obstruction II" on Oct. 24, at 10:09 am PST, at the Transcatheter Cardiovascular Therapeutics (TCT) conference in San Francisco, in the Moderated Abstracts Station 2 Emerging Clinical Science & Research, Hall A, Exhibition Level, Moscone South, Moscone Center.
The single-use, dual-lumen, non-steerable Dib UltraNav System intravascular catheter and handle received FDA 510(k) clearance in March 2022. It is compatible with frequently used needles, RF wires, and various ICE catheters. It has a broad indication for use for puncture of the septum and transport of ICE catheters to the left atrium and is applicable for any septal or left heart procedures, including appendage closure procedures, valve interventions, and catheter-based ablation.
For more information: https://fmgmedical.com/


 June 13, 2024
June 13, 2024