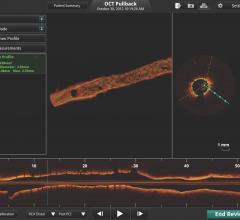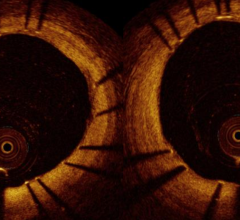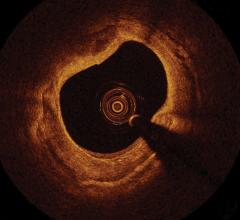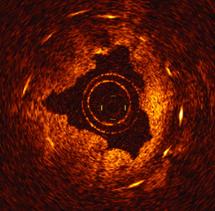
June 1, 2011 – If at first you don’t succeed, try, try again. That is what St. Jude Medical is doing after one court rejected its claims that Volcano Corp. developed an optical coherence tomography (OCT) intravascular imaging system using technology it took from St. Jude.
Earlier this year, the Massachusetts Superior Court rejected claims by LightLab Imaging, a wholly-owned subsidiary of St. Jude Medical, that Volcano’s wholly-owned subsidiary, Axsun Technologies, misappropriated LightLab's confidential OCT system information for use in the development of Volcano's (OCT) system. Axsun manufactures components for LightLab’s OCT system.
Now LightLab is bringing similar claims in a new lawsuit filed May 24 in a Delaware court, in which LightLab alleges that Axsun is wrongfully participating in Volcano's development of an alternative OCT light source, including initiatives underway with third parties. Volcano issued a statement this week denying the claims, calling them “unfounded allegations” and saying that the latest lawsuit has no merit.
Volcano has CE mark for an OCT system in Europe and said it eventually wants to bring the technology to the United States. St. Jude purchased LightLab in the spring of 2010 just after LightLab became the first company in the United States to gain U.S. Food and Drug Administration (FDA) approval for an intravascular OCT system.
OCT offers higher resolution than intravascular ultrasound (IVUS), which is the current standard for intravascular imaging used in coronary and peripheral vessel interventional procedures.

 August 29, 2023
August 29, 2023