August 12, 2013 — Ambio Health, a healthcare technology company specializing in health monitoring systems, announced its Ambio remote health monitoring system, a wireless remote health and activity monitoring tool, received Class II 510(k) clearance from the U.S. Food and Drug Administration (FDA). The system can track health information for patients with high blood pressure, heart disease and diabetes.
“Our goal at Ambio is to enable individuals to take health readings at home and get connected to health care providers, loved ones and caregivers who may not be able to visit every day,” said Kevin Jones, CEO at Ambio Health. “We are very pleased to have realized the important milestone of FDA 510(k) clearance so quickly, as it means we will be able to make this product readily available to those who need it.”
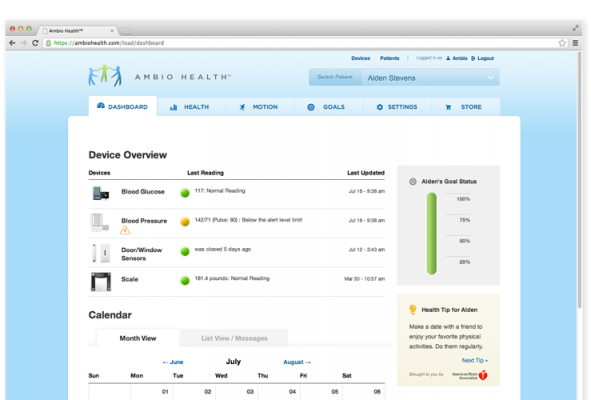
The Ambio remote health monitoring system was launched in January 2013 at the Consumer Electronics Show and named as one of eWeek’s “10 Health and Fitness Tools To Track Exercise, Chronic Conditions.” The system offers patients:
- Home health readings that are automatically logged and can be viewed by family members and caregivers from the secure, Web-based Ambio Care Portal;
- Accurate and complete readings that are available to healthcare providers for the regular monitoring of patient health;
- The ability to set individualized alert levels and notifications of exception conditions;
- Individualized reminders for readings and medications that can be sent to the patient via telephone call, text message or email;
- An affordable system designed to work with a number of health monitors; and
- A HIPAA compliant tool that keeps health information private and secure.
The system’s price starts at $49.97 for a single device, which includes a wireless glucose meter, and the user then pays an annual subscription that is the equivalent of $4.99 per month. Additional devices with wireless connectivity include a blood pressure meter and a weight scale and can be added à la carte.
Jason C. Baker, M.D., with Weill Cornell Physicians, noted: “Ambio is uniquely designed to also provide an interface on the critical measures of blood pressure and weight, which are key pieces in controlling the diabetes puzzle. The Ambio dashboard provides a comprehensive, yet straight-forward, overview of glucose control and can be personalized to meet the needs of individual patients and providers.”
For more information: www.ambiohealth.com


 October 21, 2025
October 21, 2025 









