January 31, 2011 – A new catheter used to treat coronary artery disease has received regulatory clearance in the United States and approval in Japan. The Trek and Mini-Trek systems, by Abbott, are used in angioplasty procedures and are designed to enable interventional cardiologists to open patients' narrowed coronary arteries.
The Trek system received the CE mark in May 2010.
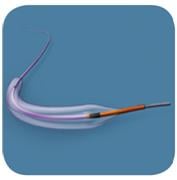
Balloon dilatation catheters help physicians open up, or pre-dilate, a lesion before a stent is placed. Evidence shows this step improves patient outcomes when the stent is later expanded in the vessel. The system was designed to help physicians treat tortuous anatomy, with the goal of addressing complex lesions in narrowed and difficult-to-dilate vessels.
"It can take multiple attempts to find the right catheter that is flexible enough to travel through twisting blood vessels and streamlined enough to push through calcified lesions, which can lead to prolonged procedure times," said Etsuo Tsuchikane, M.D., co-director of cardiovascular medicine at the Toyohashi Heart Center in Japan. "The Trek balloon is designed to allow physicians to more easily and accurately place the balloon, restore blood flow and quickly bring relief to the patient."
The family of balloon catheters incorporates several design and technology changes that have the potential to improve deliverability and performance. The system is available in two catheter designs, including the specialty catheter Mini-Trek (available in the U.S. in 1.50 to 2.0 mm sizes and available in Japan in 1.20 to 2.0 mm sizes) for accessing smaller vessels. Trek is available in more than 70 sizes and in a wide variety of diameters (from 1.2 mm to 5 mm) and lengths (from 6 mm to 30 mm).
For more information: www.abbott.com

