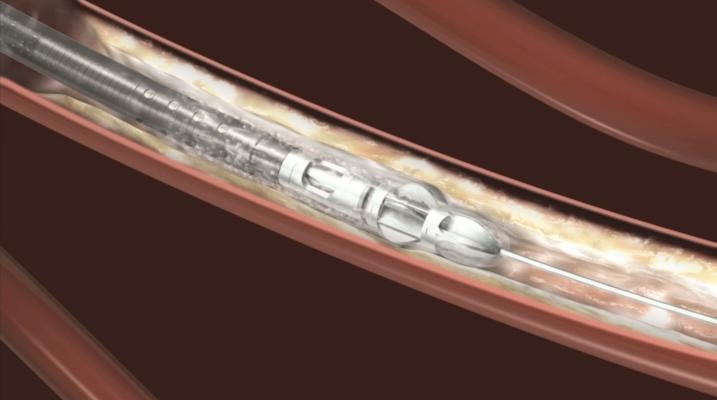
August 29, 2013 — Bayer HealthCare announced that the U.S. Food and Drug Administration (FDA) has cleared two new Jetstream atherectomy catheters. The SC (Single Cutter) atherectomy catheter is available in 1.6-mm and 1.85-mm cutting diameters. The XC (eXpandable cutter) atherectomy catheter features both front cutting tip and expandable blade technology for use in larger vessels. The XC is available in two cutting diameters: 2.1-mm blades down / 3.0-mm blades up and 2.4-mm blades down / 3.4-mm blades up.
Redesigned, integrated control pods feature:
- Ergonomic design and smaller size (32 percent) for improved ease of use
- New user interface to facilitate single or dual operator use
- Simplified guidewire management through improved wire GARD
- Reduced storage space requirements and environmental impact through new control pod design and improved packaging
The Jetstream Atherectomy System is one of the most versatile debulking solutions for the treatment of peripheral artery disease (PAD). The system catheters can be used to treat multiple lesion morphologies – from thrombus to the most challenging calcium deposits. Catheters are available in both SC and XC sizes to support treatment of vessels above and below the knee. The Jetstream System consists of a single-use catheter with control pod and a reusable, compact console that mounts to a standard IV stand.
For more information: www.ri.bayer.com


 November 14, 2025
November 14, 2025 









